
Simplified posterior urethroplasty utilizing laparoscopic instrumentation
Introduction
Posterior urethral stricture disease presents challenges for even the most skilled reconstructive urologists. The narrow channel between the pubic rami laterally, the symphysis pubis anteriorly, and the rectum posteriorly limits space for both visualization and precise suturing. Two predominant surgical techniques are used to address posterior urethral strictures. The transecting urethroplasty is a more technically simple operation that is appropriate for strictures obliterating the membranous urethra (1). A less commonly performed but also less morbid technique is the buccal mucosa graft urethroplasty (BMGU). Regardless of the surgical technique used, these are complex operations that occur in hard-to-access locations.
In order to simplify these deep operations, some authors have turned to robotic assistance to facilitate a more ergonomic operation (2). The utilization of the robot for an air-docked posterior urethral repair brings added visualization and improved ergonomics at the expense of added procedural costs and surgical time. To ease deep suture passage, Blakely et al. used a long narrow cardiothoracic needle driver to grasp a needle thrown in the proximal urethrotomy (3). Schardein et al. introduced a “sewing machine” to aid in speed during the quilting of the deep graft to the underlying dorsal bed (4). The above techniques require nuanced skill and add significant layers of complexity to already difficult cases.
Laparoscopic instrumentation can simplify urethroplasties. For BMGU, Joshi et al. used the Absorbatack™ (Medtronic, Minneapolis, MN), an absorbable polyglactin tack, to secure the deep portion of the graft in position (5). This allows fixation of the proximal portion of the dorsal graft in the setting of dorsal onlay bulbar BMGU. The disadvantage of the Absorbatack™ is that it loses fixation ability if the point of entry is not 90 degrees to the surface of the graft (6). An alternative absorbable graft fixation is the Securestrap® (Ethicon™ Somerville, NJ, USA), which can hold the tensile strength at an oblique angle (6). The Securestrap® within our institution is $325 per device. This has a more favorable application when the angle of entry cannot be at 90 degrees as is usually the case in the deep membranous urethra. While Securestrap® is useful for graft fixation, initial sutures of graft to the proximal aspect of the urethrotomy remain challenging. This step of the operation typically requires extremely difficult suture placement in a narrow channel. The RD-180® (LSI solutions, Rochester, NY, USA) offers a possible solution as the only 5 mm end-firing laparoscopic suture device. The RD-180® costs $225 per device with each Monoglide suture costing $34 per suture. It has been previously employed for transurethral endoscopic procedures (7,8) but not for open urologic procedures. Herein we describe the use of a novel combination of laparoscopic instrumentation to simplify posterior urethral reconstruction. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/tau-21-498).
Methods
An Institutional Review Board approved retrospective database of posterior urethral stricture repairs was reviewed between October 2016 and October 2020. We identified patients undergoing a posterior urethral stricture repair utilizing a combination of the RD-180® suture device and the Securestrap®. Patients with greater than or equal to 4 months of follow up were included in the analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The study was approved by the Ethics Committee of City of Hope National Medical Center (No. 15436) and informed consent was taken from all the patients.
Technique selection
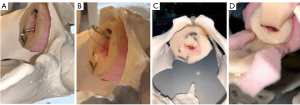
In cases of complete urethral obliteration, we perform a non-transecting excision and primary anastomosis (EPA) while preserving the bulbar arteries. The exception is in revision cases where excision of a previously transected urethra is required. In this case a traditional transecting EPA is performed. In cases in which there is stenosis, but a viable lumen and the prostate is still present, we prefer a ventral onlay BMGU to eliminate dorsal urethral dissection in case a future artificial urinary sphincter (AUS) is needed. If there is no prostate, we prefer the dorsal BMGU, in order to prevent potential rectal injury (Figure 1). Patients are counseled regarding the off-label use of these devices, and potential complications, particularly the long indwelling time of the sutures and tacks. This is our standard of care, however we cannot rule out bias in our patient selection.
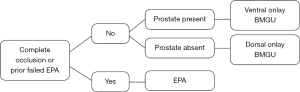
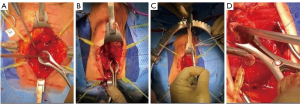
For a non-transecting EPA, we follow the procedure described by Gomez et al. (9). After the urethra is mobilized and the scarred section is excised, sparing the bulbar arteries, we use the RD-180 to pass the suture through the proximal stump beginning at the 6 and 12 o’clock positions (Figure 2). Using a free Richard-Allen needle (Aspen Surgical Products, Caledonia, MI, USA) the tail end of the monoglide® (LSI solutions, Rochester, NY, USA) suture is passed though the distal urethral stump in the corresponding location (Figure 3). This is then repeated from 5 to 1 o’clock and 7 to 11 o’clock. Leaving the suture attached to the RD-180® after each pass saves time, and we find that we can average three throws per suture. After all sutures are placed, we then tie following the pattern 6, 5, 7, 4, 8, 3, 9, 2, 10, 1, 11, and finally 12 o’clock. Two patients underwent a full transecting EPA due to prior failed EPA resulting in bulbar necrosis.
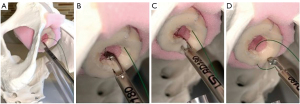
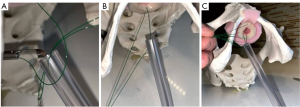
In the setting of a dorsal or ventral onlay buccal mucosal urethroplasty, we utilize the approach described by Blakely et al. (10) or Barbagli et al. (11), respectively. In the setting of ventral onlay, we place 3–5 interrupted sutures in the proximal ventral urethrotomy with the RD-180®. The device allows for easy passage of a needle out-to-in on the ventral urethrotomy, leaving the tail of the suture in-to-out. Then we pass the needle in-to-out of the graft, so the ultimate knot will be within the lumen of the urethra. Often, when working deep in the pelvis, we will use a laparoscopic knot pusher to pass knots deep into the operative field.
After the 3–5 proximal stay sutures are placed, the Securestrap® is used to secure the graft to the underlying prostate and muscle tissue. Next, 4-0 PDS® (Ethicon™ Somerville, NJ, USA) sutures are placed on either side of the proximal urethrotomy, thrown to the graft and ran closed over a 16-French catheter. When performing a dorsal onlay BMGU (Figures 4-6), a similar approach is utilized to pass the deep suture and deploy the graft. Once secured proximally the Securestrap® is used to quilt the graft to the dorsal bed, weather through the membranous urethra or the proximal bulbar urethra. We avoid the Securestrap® in the distal bulb and penile urethra as hand thrown quilting sutures are easily performed. Once quilting is complete, we will sew the left and right urethrotomies to the graft over a 16-French silicone catheter.
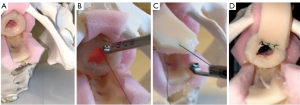
The catheter is removed in 4 weeks with a peri-catheter retrograde urethrogram. All patients are then seen at 4 months with cystoscopy and followed annually thereafter. International prostate symptoms score (IPSS) was performed on all patients at each visit. Failure is defined as inability to pass a 16 French cystoscope through the region of repair.
Statistical analysis
Unpaired Student’s t-test with 95% confidence interval was used to evaluate pre and post-operative IPSS and BI using GraphPad statistical software (GraphPad, San Diego, CA, USA).
Results
From October 2016 to October 2020, 20 patients underwent posterior urethral stricture repair using these laparoscopic instruments. Median age was 70 years (28–90 years). Median follow up was 12 months (5–50 months) (Table 1).
Table 1
| Items | Value |
|---|---|
| Age (years), median [range] | 70 [28–90] |
| Follow up months, median [range] | 12 [5–50] |
| Prior intervention | 20 |
| EPA | 4 |
| Dorsal onlay | 10 |
| Ventral onlay | 6 |
| Stricture length (cm), mean [range] | 3 [1.5–16] |
| Operative time (min), median [range] | 150 [120–180] |
| Stricture etiology | |
| Unknown | 6 |
| Radiation | 10 |
| Enlarged prostate surgery | 3 |
| Pelvic fracture urethral injury | 1 |
| Stricture location | |
| Bulbomembranous | 14 |
| Isolated membranous | 2 |
| Bladder neck and membranous | 1 |
| Panurethral and membranous | 2 |
| Pelvic fracture urethral injury | 1 |
All patients had prior interventions: 14 patients had prior dilations, and 6 had prior urethroplasty: including one with prior staged bulbar urethroplasty, and two with prior failed EPA. Four patients underwent EPA, 10 underwent dorsal onlay, and 6 underwent ventral onlay. Mean stricture length was 3 cm (1.5–16 cm). Median and mean operative time was 150 minutes (120–180 minutes). No peripheral neuropathies or positional injuries were noted. With failure defined as inability to pass a 16 Fr scope, success rate was 95% (19/20 patients).
Post-operative morbidity included fistula formation in 2 patients. One patient with a panurethral stricture developed a distal penile fistula after a combined membranous urethral stricture repair and panurethral stricture repair managed with a single sided dorsal onlay urethroplasty via a penoscrotal invagination approach. Another with a dorsal onlay developed a perineal fistula. Each was successfully managed with prolonged Foley catheter drainage.
Preoperative international prostate symptom score (IPSS) and bother index (BI) was collectable in 13 patients. The remaining 7 patients had indwelling suprapubic catheters. Median IPSS was 15 [0–35]. Median BI was 4 [2–6]. Post-operative IPSS at last follow up was 6 [2–29] and BI was 3 [0–4]. Preoperative and post-operative IPSS and BI were significantly improved (P<0.05). There were 2 cases of de novo incontinence, each occurring in the redo EPA patients. No patients suffered de novo incontinence after either dorsal or ventral BMGU. One patient had a persistent Securestrap® tack in place at 4 months, and this was removed with a grasper.
Discussion
This novel combination of laparoscopic instrumentations dramatically eases time-consuming ergonomic challenges of posterior urethral stricture repair. These instruments make an otherwise very difficult operation easily reproduceable and durable repair, even in the deepest of urethral strictures. Specifically, these instruments confer advantages in placing proximal sutures and also deep quilting sutures. We found these devices easy to use with predictable and reproducible quality of sutures placed with the RD-180® as well as the Securestrap® with a learning curve of only one case for our fellows.
The placement of proximal sutures in a very narrow space is a major disadvantage of posterior BMGU. Working in a deep channel within a urethrotomy offers a limited field of view compared to a widely resected EPA. Also, dorsal quilting sutures are particularly difficult to place. Blakely et al used a narrow cardiothoracic needle driver to grab a suture that has been passed with a curved needle driver (3). We found that passing a deep suture, at the level of the bladder neck for example, to be challenging with this approach. We have not pursued air-docking robotic techniques due to added time and cost. We have found the low profile 5 mm RD-180® has made these throws simple and reliable, even if working to the level of the bladder neck. While Blakely et al and others have proposed innovative solutions to this problem, none offer such a simplified approach that also substantially reduces operative times and complexity. Our operative times described herein are on average about 30 minutes faster than those described using cardiothoracic needle drivers.
Another complexity of BMGU is securing the graft to underlying muscle tissue. The use of Securestrap® described in our series added to the RD-180 significantly decreased operative times and enhanced ease of procedure. Others have innovated this portion of the procedure as well but with modifications that still demand more skill and time. To quilt the graft down, Schardein et al. described a surgical “sewing machine”, using a barbed suture placed through a spinal needle (4). Passing the suture in and out of the graft will allow the barbs to anchor the graft to the underlying tissue. We have had trouble in the dorsal onlay having enough depth to allow the suture to hold adequately. We looked at the work of Joshi et al., using the Absorbatack™ (Medtronic, Minneapolis, MN, USA) to secure the graft in place (5). While they report good outcomes, the Absorbatack™ works best at 90-degree angle of entry-losing tension if it goes in at an oblique angle. Perhaps this is why they limited the use to bulbar urethra where the perpendicular angle of entry is possible. We favor the Securestrap® by Ethicon™, which holds similar tension to the Absorbatack™, even at a less than 90 degree entry. Using the Securestrap® for the deep urethroplasty helps give a reliable fixation even when we enter at the necessary steep angle of entry. Importantly, we do not favor using the Securestrap® in the distal bulb or penile urethra where the normal quilting sutures can be performed easily.
Some might argue that while we are saving time by BMGU standards, EPA is still a more efficient operation. Comparing our operative time with other posterior urethral stricture repairs shows that for a large contemporary multi-institutional series on posterior EPA, the mean operative time was 176 minutes, and the median was 150 (1). For a similar multi-institutional series for dorsal onlay BMGU the median operative time was 184 minutes (12). Whereas the robotic deep urethroplasty has a mean operative time of 240 minutes (2). While ours is not a homogenous population, including EPA, ventral BMGU, and dorsal BMGU; there was no trend favoring one or the other technique in terms of time in our series. In particular, there was no advantage in the EPA population. When no graft is needed it makes sense that the operating room time is better with EPA. In our series however, 2 of the 4 EPA patients were redo cases likely influencing the operative time to match that of the other patients. Despite these findings, the precision of the RD-180®, for EPA or BMGU, in making deep perineal throws has rendered it an invaluable time saving part of our reconstructive armamentarium.
Limitations
There are several key limitations to this work. This is a small, retrospective feasibility series intended to introduce new, time saving techniques into the field of posterior urethroplasty. With regard to the employment of these devices, we find the biggest critiques to be that 2-0 suture required by the RD-180® platform is too large. For instance, we found ensuring that the knots of the 2-0 are within the lumen of the urethra helps prevent any issue with the graft sitting on the bed. We found the use of 2-0 in the EPA to be reassuring and thus far having no significant consequence—and in particular simplifies the non-transecting EPA. Another barrier to wide adoption of this technique in under-resourced settings is cost. We would counter that if one assumes a modest $37.45 cost per minute of operating room time (2), the devices are paid for in roughly 14 minutes of time saved.
Conclusions
The combination of the RD-180® and the Securestrap® has become essential to our posterior urethral stricture repair armamentarium. Further data and longer follow up is needed to confirm these reliable outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/tau-21-498
Data Sharing Statement: available at https://dx.doi.org/10.21037/tau-21-498
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tau-21-498). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of City of Hope National Medical Center (No. 15436) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Voelzke BB, Leddy LS, Myers JB, et al. Multi-institutional Outcomes and Associations After Excision and Primary Anastomosis for Radiotherapy-associated Bulbomembranous Urethral Stenoses Following Prostate Cancer Treatment. Urology 2021;152:117-22. [Crossref] [PubMed]
- Unterberg SH, Patel SH, Fuller TW, et al. Robotic-assisted Proximal Perineal Urethroplasty: Improving Visualization and Ergonomics. Urology 2019;125:230-3. [Crossref] [PubMed]
- Blakely S, Kaefer D, Daugherty M, et al. Membranous urethroplasty using dorsal onlay buccal mucosal graft for strictures associated with turp or radiation therapy. The Journal of Urology 2017. Available online:
10.1016/j.juro.2017.02.235 10.1016/j.juro.2017.02.235 - Schardein J, Scott KA, Bratslavsky G, et al. A surgical “sewing machine” for rapid graft quilting and suturing in challenging spaces. Urology Video Journal 2020. Available online:
10.1016/j.urolvj.2020.100027 10.1016/j.urolvj.2020.100027 - Joshi PM, Raveenthiran S, Desai D, et al. Vicryl Tack for graft fixation during bulbar urethroplasty. Trends in Urology and Men's Health 2019:26-8.
- Cardinale M, Jacinto G, Cohn S, et al. Comparison of Acute Holding Strength of an Absorbable Strap Fixation Device in Porcine Flank at Various Implantation Angles.
- Humphreys MR, Sauer JS, Ryan AR, et al. Natural orifice transluminal endoscopic radical prostatectomy: initial perioperative and pathologic results. Urology 2011;78:1211-7. [Crossref] [PubMed]
- Oliveira C, Autorino R, Ferreira C, et al. Novel method of full-thickness bladder closure with an endoscopic suturing machine: a survival study in a porcine model. BJU Int 2015;115:330-5. [Crossref] [PubMed]
- Gomez RG, Campos RA, Velarde LG. Reconstruction of Pelvic Fracture Urethral Injuries With Sparing of the Bulbar Arteries. Urology 2016;88:207-12. [Crossref] [PubMed]
- Blakely S, Caza T, Landas S, et al. Dorsal Onlay Urethroplasty for Membranous Urethral Strictures: Urinary and Erectile Functional Outcomes. J Urol 2016;195:1501-7. [Crossref] [PubMed]
- Barbagli G, Kulkarni SB, Joshi PM, et al. Repair of sphincter urethral strictures preserving urinary continence: surgical technique and outcomes. World J Urol 2019;37:2473-9. [Crossref] [PubMed]
- Policastro CG, Simhan J, Martins FE, et al. A multi-institutional critical assessment of dorsal onlay urethroplasty for post-radiation urethral stenosis. World J Urol 2021;39:2669-75. [Crossref] [PubMed]


