Neuromodulation in neurogenic bladder
Introduction
The term “neurogenic bladder” encompasses a highly heterogeneous patient population with many different disease processes and varying clinical manifestations. Depending on the level, completeness, and pathophysiology behind each neurologic insult, patients with neurogenic bladder may have various permutations of storage and voiding disorders. Estimates of lower urinary tract dysfunction among patients with neurologic disorders range from 12–19% in patients after stroke to up to 90% in patients suffering from multiple sclerosis (MS) (1).
The management of neurogenic bladder varies depending on the predominant symptoms but may involve behavioral modification, clean intermittent catheterization, pharmacotherapy, intradetrusor onabotulinumtoxinA injections, or major reconstructive surgery including bladder augmentation and urinary diversion. Neuromodulation is a well-established treatment option for patients with non-neurogenic overactive bladder and urinary retention who have previously failed more conservative therapies, yet its applicability to the neurogenic bladder population has only recently been examined more in depth. In this article we will discuss the outcomes, contraindications, and special considerations of sacral and percutaneous tibial nerve stimulation (PTNS) in patients with neurogenic lower urinary tract dysfunction.
Neuromodulation
History
The development of neuromodulation began with direct electrical stimulation of the bladder in the late 1870s. From there researchers moved from stimulation of the target organ to stimulation of select peripheral and sacral nerves. In the early 1980s Tanagho and Schmidt began developing an implantable sacral electrode which would provide the basis for the concept of sacral neuromodulation (SNM) and the InterStim device (Medtronic) (2). Around the same time McGuire used traditional Chinese acupuncture techniques and found that electrical stimulation of the tibial nerve inhibited bladder overactivity thereby providing the concept of PTNS. Both SNM and PTNS were approved by the FDA for use in non-neurogenic overactive bladder in the late 1990s and SNM was later additionally approved for non-obstructive urinary retention. The neurogenic population was initially excluded from approval because it was thought that an intact neural system was necessary for efficacy of the devices. Since this time, however, investigators have extrapolated the use of these devices to the neurogenic bladder population in several small heterogeneous trials (1,3,4).
Physiology
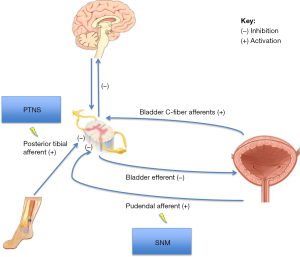
The exact mechanism of action of neuromodulation has not been clearly elucidated. In the past neuromodulation was thought to act by direct muscle stimulation. However the electrical current produced by neuromodulation is below the threshold for motor activation, thus this theory is likely inaccurate. The current leading hypothesis suggests that neuromodulation works by stimulating peripheral somatic afferent nerves (C fibers). In SNM the pudendal afferents are stimulated and in PTNS the sensory component of the tibial nerve is stimulated. Stimulation of the peripheral afferent nerve blocks competing abnormal visceral afferent signals from the bladder and prevents reflex bladder hyperactivity or retention (Figure 1) (1,4-7). However, the effects of neuromodulation are not just limited to spinal cord reflexes. Functional magnetic resonance imaging (MRI) studies demonstrate changes to brain activity in the brainstem and limbic systems with SNM and additional studies have shown differences in brain activity in acute and chronic neuromodulation consistent with sensorimotor learning (8,9).
Sacral neuromodulation (SNM)
Description of procedure
SNM placement is performed as a staged procedure in order to demonstrate efficacy in the first phase prior to permanent implantation in the second phase. In the first surgical procedure a tined quadripolar lead is percutaneously inserted into the S3 foramen using bony landmarks and fluoroscopic guidance. The nerve root is stimulated electrically to assess placement. Responses indicating proper placement include plantar flexion of the ipsilateral toes, contraction of the levator ani muscle causing “bellows”, pulling sensation in the rectum, or tingling/vibratory sensations in the vagina, labia, scrotum or penis. After placement the lead is connected to an external pulse generator. The patient wears the temporary device for approximately 1–2 weeks and has the ability to change the stimulation intensity, pulse width, and frequency of the pulse generator. If the patient has a 50% improvement or greater in symptoms during the trial period then the lead is attached to an implantable pulse generator (IPG) and the device is fixed into the upper portion of the buttock during the second surgical procedure. If the patient did not have a 50% improvement in symptoms then the lead is removed in the second procedure (4,10).
Outcomes
Prior to 2010 the literature examining the use of SNM in the neurogenic population was limited by heterogeneous patient populations including multiple neurologic diagnoses, lack of randomized controlled trials, small sample sizes, and short-term follow-up. Since this time, however, the literature has shifted somewhat to separate studies based on individual neurologic diagnoses and one randomized trial to this effect is currently underway (11). Herein, we will examine outcomes of SNM in the generalized neurogenic population and then go on to discuss efficacy of this therapy among patients with specific neurologic disorders.
General
Overall, the data on SNM in the neurogenic population is limited to patients with stroke, Parkinson’s disease, MS and incomplete spinal cord injury. With these limitations in mind, the current literature is generally positive and indicates that SNM demonstrates similar efficacy among the neurogenic and non-neurogenic populations in terms of successful test phase, device implantation, clinical outcomes, urodynamic outcomes, quality of life data, and safety among carefully selected cases. Per the literature, patients with neurologic disorders demonstrate test phase success rates that range from 50–68% and implantation success rates that range from 80–92%, comparable to implantation success rates of 80–90% in the non-neurogenic population (3,7,12,13).
Two studies, neither of which defined the severity of the neurologic insult, looked at neurogenic overactive bladder patients with stroke, Parkinson’s disease, MS, among other neurologic conditions, and showed durable decreases in frequency, incontinence episodes, and urgency symptoms over time. These same patients, however, had significantly lower decreases in number of voids per day compared to the non-neurogenic population (Medtronic, Minneapolis, Minnesota, USA) (1 vs. 3) (13,14). Another study evaluated 39 patients with neurogenic overactivity due to mixed etiologies [primarily representing spinal cord injured patients described as American Spinal Injury Association (ASIA) C and D, who have higher functional status than ASIA A and B individuals], and demonstrated that 43% of patients were able to stop their anticholinergic medications entirely and 80% of patients reported complete continence by bladder diary with SNM (15).
The same study also evaluated 11 highly functional spinal cord injured patients with urinary retention and found that all subjects were able to void spontaneously after device implantation (15). This neurogenic group, along with another study involving both complete and incomplete spinal cord injured patients, also demonstrated sustained improvement in urodynamic parameters with increased bladder capacity, improved compliance, and increased maximum detrusor pressure (15,16).
While both neurogenic and non-neurogenic groups demonstrate statistically significant improvement in most quality of life measures after device implantation, those with a neurologic diagnoses of stroke, Parkinson’s disease, and MS have been shown to have worse baseline physical scores that slightly declined in those with progressive disease (13). In long-term follow-up among patients with various types of neurologic disorders, implant success appeared durable over time with 75.7% having continued success at 4 years (14). In terms of safety, there did not appear to be any increased risk of complications in the neurogenic population (12.9–24% neurogenic vs. 33% non-neurogenic) (7,12-14).
While these results may sound promising, it is important to consider the specifics of each publication separately before drawing any generalizable conclusions. Unfortunately, most studies involving neurogenic patients do not include descriptions or indicators of disease severity, which makes their interpretation and significance difficult to understand. Descriptions of ASIA classification, an indicator of the extent and severity of the neurologic insult, is imperative to understanding the population studied, but is often not provided. For example, a fully ambulatory individual with MS may be expected to have a different response to therapy than an individual with MS who uses a wheelchair. However, this level of detail is often not communicated in the literature. Furthermore, the findings of each of the studies discussed herein should be interpreted with these limitations in mind.
Multiple sclerosis (MS)
Particular attention has been given to the outcomes of SNM in the MS population. This population presents unique challenges given the heterogeneity of neurologic lesions (and thus symptoms) and the ability of the disease to progress over time. Generalized test phase success rates have been reported to be approximately 50–84%, comparable to success rates in the broader neurogenic bladder population (3,7,14,17). Interestingly, while success has not been found to correlate with duration of the neurologic disease nor the specific urologic symptoms, multiple studies cite significant increases in quality of life measures with patient satisfaction up to 75–86% (17-19).
One study of 25 individuals with MS (severity of disease not described) found that 100% of subjects that failed the test phase had symptoms of urinary retention, as opposed to overactivity, suggesting that SNM be better utilized for treatment of bladder overactivity in this population (17). Of those MS subjects with detrusor overactivity, SNM has been found to reduce frequency by 6–9 voids per day, reduce incontinence by 4–10 episodes per day, and increase voided volume by 77–84 mL per void (1,17,19).
In MS subjects with detrusor-sphincter dyssynergia, which is not an FDA approved indication for SNM, SNM was associated with a significant increase in voids (5–8 per day), with increase in voided volume by 154 mL, and with a decrease in the number of catheterizations by two per day (17).
Another study of 14 subjects with MS (of varying types: three benign disease, seven relapsing remitting, four secondary progressive) and urinary retention found that 86% of individuals were able to void spontaneously after IPG placement with mean post-operative post-void residual of 51 mL and max flow rate of 18 mL/s (18). This study goes on to suggest that in this population, individuals with two or more leads producing bellow or plantar flexion at an IPG power rating of six or less have a 76% chance of success.
Failure rates due to disease progression ranged from 16–33% with follow-up extending 0.5–4.3 years (1,4-7,19). One study found that failures occurred on average approximately 12 months after SNM placement (14). Two studies found slightly lower mean battery life 5–6.1 years in the MS population which could potentially be due to higher amplitude usage as the disease progresses, ultimately leading to an increase in revision rates (17,18).
Patients with neurologic disease or injuries that have the potential to change over time, such as MS, need to understand that their SNM device may lose efficacy as the natural history of their disease progresses. Additionally, the need for possible MRI imaging should be taken into account prior to considering SNM placement in these individuals. Some authors recommend proceeding with SNM only in the relapsing-remitting MS subtype who has not experienced any relapse for 2 years who are not likely to require repeated MRI scans (3,7,12-14,19).
Spinal cord lesions
Similar to the literature on MS, the outcomes of SNM in individuals with spinal cord lesions are quite difficult to interpret based on the population studied. The majority of studies combine both incomplete and complete lesions from a variety of etiologies, lack descriptions of the extent and severity of disease or only include the least severe patients (classified as ASIS C or D), and are thus highly heterogeneous. Overall, success rates range from 29–70%, depending on the study and the population examined (7,13,14,20,21).
One meta-analysis found that patients with any type of spinal cord lesion demonstrated a test phase success rate of 35% (range, 29–44%), depending on the type of lesion (success rates were 29%, 40%, and 44% for incomplete, complete, and unknown types of lesions) (7,15). They further found that the success rates of the permanent implant at time of last follow-up was 77%, which was lower than in the success rate for all types of neurogenic bladder combined (92%). Again success is this population was variable and depended on the type of spinal cord lesion (success rates were 83%, 81%, and 58% for incomplete, complete, and unknown types of lesions) (7,14). This study demonstrated high inter-study heterogeneity combined with a lack of detail on disease severity, and results should be interpreted with this in mind.
One study of 85 patients with incomplete spinal cord injuries (73% traumatic, 25% myelitis, 3% vascular) and non-obstructive urinary retention demonstrated a test phase success rate of 43% and these patients subsequently underwent permanent IPG placement. Of note, this study was restricted to higher functioning patients, classified as ASIA C or D. After the test phase in subjects with retention, 22 additional subjects (26%) were able to void spontaneously with a significant increase in maximum flow rate and a significant decrease in post-void residual. A significant predictor of successful first stage response was the first sensation of bladder filling (47% vs. 13%, P=0.02) on urodynamics. Thirty-four patients had adequate follow-up and of those 67.6% achieved consistent success with the permanent implant. The remaining third of patients had a least one or more “failures” during follow-up that required contralateral or S4 implantation. After re-implantation all patients subsequently improved both subjectively and on urodynamics. Of the failures, one occurred before 3 years, five occurred between 3–5 years and the remaining seven occurred after 5 years. The cause of failure was often not known, with displacement of the lead representing a minority of cases. The author suggested “nerve fatigue” as a possible source of failure as all subjects who failed their initial implants achieved amelioration of symptoms with a new contralateral implant (20).
Another study incorporated 23 individuals with mostly incomplete (91%) spinal cord injuries from various heterogeneous etiologies (myelomeningocele: 9, incomplete SCI: 7, spina bifida: 3, complete SCI: 2, resection of cord tumor: 1, intravertebral anesthetic complication: 1), ASIA classification not reported. This study found that test phase success rate of 56.5%. They found that test phase outcomes correlated best with symptoms, as patients with urgency-frequency or urinary incontinence had better outcomes (65%, 70% respectively) than those with dysuria (29%). A significant positive predictor of test phase success was lower volume at first sensation of bladder filling on baseline urodynamic parameters (361 vs. 421 mL at baseline) and a non-significant negative predictor was a complete spinal cord lesion (21). Ultimately they performed an implant in 13 patients (five of which had incomplete spinal cord injuries, six had myelomeningocele, one had spina bifida, and one had post resection of spinal cord tumor) and had a permanent implant success rate of 92.3% at 17.5 months of follow-up. Patients experienced significant reduction in number of voids, episodes of urgency and degree in urgency with increase in volume per void (21).
Interestingly, recent work supported by Medtronic, the company who makes InterStim, or sacral neuromodulators, has shown potential utility of early SNM during the spinal cord shock phase after complete spinal cord lesions to T2-11, ASIA A classification. This study compared ten subjects who elected to undergo bilateral SNM within 4.5 months of injury to six similarly injured subjects in a control group. At a mean of 26.2 months of follow-up, individuals in the SNM group had favorable findings compared to those in the control group, despite use of pharmacotherapy in the latter group. Namely, no individuals in the SNM group developed detrusor overactivity on urodynamics, poor compliance with detrusor pressures over 30 cmH2O, or urinary incontinence. Additionally, the SNM group demonstrated decreased infection rates, better quality of life, and improved erectile and bowel function. These results are intriguing and need to be reproduced in a larger more vigorous trials prior to being utilized en mass (22).
Spinal surgery
A retrospective review examined the use of SNM for approved indications in 32 individuals who had undergone spinal surgery at various levels (67% performed for disc disease) and compared outcomes to 102 non-neurogenic controls. Sixty-three percent of neurogenic subjects had a successful test phase and underwent permanent implantation compared to 75% of the control group. When comparing groups based on urinary symptoms, there were similar test phase outcomes except in the setting of urgency urinary incontinence, wherein the neurogenic group was significantly less likely to undergo a permanent implant (58.8% vs. 89.5%, P=0.002). Explantation rates and time to explantation were similar between neurogenic and non-neurogenic populations (23).
Cerebral palsy
A limited number of studies, mostly case reports, have examined the use of SNM in patients with cerebral palsy (13,14,24,25). Test phase success rates and implant success rates are very high (100% for both), however, these results are based on small case numbers and are in very well selected ambulatory patients with minimal medical co-morbidities and good sensation on urodynamic testing (7,25).
Relative and absolute contraindications
While SNM may be effective in a select group of more functional neurologic patients with overactive bladder symptoms and urinary retention, there are several indications where SNM is not approved or is contraindicated. SNM has not been shown to be effective for stress incontinence or mixed urinary incontinence (5,17-19). Additionally, older age has been shown to be associated with lower rates of efficacy. In patients with urgency urinary incontinence in both the neurogenic and non-neurogenic populations, subjects over the age of 55 had lower cure rates (defined as no pads per day) compared to younger subjects (65% vs. 37%, P<0.05 in non-neurogenic, 56% vs. 29% in neurogenic). Also, both younger and older patients with more than three co-morbidities had a decreased chance of cure (defined as no pads per day) (17,26). One study looked at SNM in various neurogenic populations and found that non-ambulatory patients have worse outcomes (18,25). Additionally, significant spinal abnormalities or contractures may lead to difficult lead placement and pressure on IPG may cause or exacerbate sacral decubitus ulcers.
SNM should not be performed in pregnant women due to theoretical risk of fetal loss or preterm labor. MRI of the abdomen or pelvis is contraindicated due to concerns of device dislodgement, heating of the electrodes and changes to the program due to the magnetic fields. New studies have demonstrated that head-only 1.5 tesla MRI examinations can be safely performed with newer generation IPG models (27,28).
Additional benefits
SNM additionally appears to have a positive effect on both bowel and erectile function. One study of six individuals with incomplete spinal cord lesions with both urgency urinary and fecal incontinence responded positively to SNM with all regaining fecal continence post-operatively in addition to significant improvements in bladder diary and urodynamic findings. Two subjects with baseline constipation as well as lower urinary tract dysfunction had 50% improvement in bowel symptoms after implantation. Finally six individuals undergoing SNM implantation for neurogenic urinary retention had erectile dysfunction managed with oral medications. Post-implantation the average Sexual Health Inventory for Men (SHIM) scores improved from 15.2 to 22 and all subjects were able to stop oral erectile dysfunction medications (29).
Percutaneous tibial neuromodulation (PTNS)
Description of procedure
PTNS is an alternative neuromodulation modality for patients who may not be good candidates for or who may not want to undergo SNM. There are several variations on how to perform the needle placement. One such variation is with the patient sitting in a frog-leg position with the knees flexed and the soles of the feet touching. A 34-gauge stainless steel needle is inserted three fingerbreadths cephalad from the medial malleolus and just one fingerbreadth posterior to the margin of the tibia at an angle of 60 degrees. The needle is advanced 3–4 cm posterior to the tibia. A stick-on electrode is placed on the medial surface of the calcaneus or on the bottom of the foot. A stimulator is connected to the needle and to the grounding pad. The needle is stimulated and proper placement is demonstrated by either a sensory (tingling sensation in ankle, foot or toes) or motor response (great toe flexion and/or fanning or plantar toe flexion of toes 2 through 5) (30).
Stimulation is titrated based on patient pain. Therapeutic sessions are typically once per week for 12 weeks. If patients have a good response they are offered maintenance therapy. One study found that in individuals with MS the presence of a sensory response, either with or without a motor response during stimulation, was associated with a better treatment outcome (P=0.001) and that the interval maintenance needed was every 2 weeks in 60% of subjects (4,31,32).
Outcomes
General
As in SNM, the literature reports of PTNS in the neurogenic bladder population is complicated by limited number of studies, small sample sizes, non-standard treatment plans, poor descriptions of the extent and severity of neurologic disease, and heterogeneous patient populations. With this in mind, a recent meta-analysis examined the success of PTNS in a variety of different patient populations, including MS and Parkinson’s disease, with mixed findings on success rates ranging from approximately 40% to 100% for neurogenic overactive bladder or urinary retention (33). The broad ranges of success are confounded by varying neurogenic patient populations and differing definitions of success used in each study, including clinical, urodynamic or quality of life parameters. Another study examined urodynamic outcomes in a mostly neurogenic population with detrusor overactivity (MS: 13, spinal cord injury: 15, Parkinson’s: 9, idiopathic: 7) and determined that PTNS caused a delay in onset of involuntary detrusor contractions by 69 mL during the filling phase and an increase in bladder capacity by 56 mL and was considered to be “positive” in 50% of patients (34).
Multiple sclerosis (MS)
The majority of PTNS research in neurogenic bladder has focused on the MS population. As previously described, this population is unique in that patients have mixed urologic diagnoses including detrusor over-activity, detrusor-sphincter dyssynergia, or detrusor underactivity. One study looked at 18 individuals with MS and lower urinary tract symptoms (LUTS) treated with PTNS. Ten (54%) had relapsing remitting, seven had secondary progressive and one had primary progressive disease. No patients had signs of peripheral neuropathy. At 3-month follow-up 89% of subjects were subjectively pleased with the results [assessed via patient perception of bladder condition (PPBC) questionnaire and visual analogue scale]. Decreases in urinary incontinence were not statistically significant. Of note, one patient with advanced disability from secondary progressive MS and another patient with relapsing remitting MS without disability and detrusor sphincter dyssynergia showed no response to treatment (35).
Another study of 70 ambulatory MS patients who were free from MS relapse during the preceding 3 months and who had refractory overactive bladder symptoms were treated with PTNS. Specifically, 82.6% of subjects cited improved urgency, 51.3% reported resolution of urgency, 66.7% reported improved frequency, 62% reported improved continence, and 44.9% reported resolution of incontinence. Quantitatively subjects on average experienced 2–6 fewer daytime voids, 2–3 fewer night-time voids, and 2.7 fewer incontinence episodes per week. These findings correlated with average increased voided volumes by 43–89 mL and decreased post-void residuals by 16–55 mL (36).
While some studies have reported increased bladder capacity and increased volume at signs of the first detrusor contraction, these findings have not been duplicated across studies nor do they appear to be associated with patient subjective response (36-38). One study of MS patients with all types of disease (51% relapsing remitting) suggested that despite good initial outcomes, these results improve over time with overall symptom improvement increasing from 70% at 6 months to 82% at 24 months (32).
Parkinson’s disease
There is limited research on PTNS in the Parkinson’s disease population. Two studies for which incomplete data are available examined outcomes in 6 and 29 subjects, respectively, and found 83–89% improvement in subjective symptoms based on standardized patient questionnaires (39,40). Of note, one of the studies combined patients with Parkinson’s disease and multiple system atrophy (MSA). Other studies report improvement in urodynamic parameters with a 49–96 mL improvement in bladder capacity and 100 mL increase in volume before the first detrusor contraction (39,41).
Stroke
While there is only one study examining the use of PTNS in the setting of stroke, it is one of the few examples of a randomized clinical trial in this literature. In this trial 24 adult male subjects with neurogenic overactive bladder due to ischemic stroke were randomized to either PTNS twice weekly for 6 weeks versus general advice and stretching for 6 weeks. Compared to the controls, the PTNS treatment arm experienced non-significant decreases in urgency (by 25%), urgency urinary incontinence (by 8%), nocturnal enuresis (by 17%), and nocturia (by 33%). Treatment arm individuals additionally had a significant decrease in daytime frequency and significant improvement in subjective symptom scores compared to the control group. These results were maintained at 12 months of follow-up. The authors additionally concluded that patients with right hemisphere lesions, advanced age and high body mass index (BMI) had more urinary symptoms than other patients (42). It is important to remember that while these results sound promising, they include a very small number of participants and overall the findings were not statistically better than general advice and stretching.
Spinal cord injury
Only one recently published study has examined the use of PTNS in spinal cord injury patients. This study randomized 100 individuals to either PTNS or solifenacin. The majority of spinal cord lesions in this cohort were described as “complete”; however, ASIA classification was not reported. At 2 weeks there was a statistically significant improvement in the examined parameters of volume per catheterization, leakage per day, and quality of life in both the PTNS and solifenancin groups, but importantly, there was no statistically significance between treatment groups. While the treatment results were similar between groups, PTNS was noted to be more tolerable than pharmacologic management, as 5% of the solifenacin group experienced side effects and two subjects discontinued the trial for this reason (43).
Relative and absolute contraindications
Absolute contraindications for PTNS therapy include patients with pacemakers, implantable defibrillators, coagulopathy, or who are pregnant (44). In the non-neurogenic population, patients with worse obstructive symptoms, detrusor overactivity on urodynamics, and poor mental health scores on the SF-36 are associated with poor treatment outcomes (33).
Special considerations
PTNS may be a more suitable neuromodulation treatment choice in the MS population as this population is likely to need future MRI imaging and may potentially have disease progression for which a permanent implant may no longer be efficacious. PTNS is additionally a good alternative to SNM in patients with skeletal abnormalities preventing appropriate placement of the sacral neurostimulator.
Conclusions
While the current literature is optimistic about the use of SNM and PTNS in the neurogenic population, it is also complicated by a limited number of studies, small sample sizes, non-standard treatment plans, poor descriptions of patient populations and the extent and severity of neurologic disease, and heterogeneous patient populations. Further studies with larger sample sizes, better disease classification, and more vigorous study designs are warranted before more widespread use of these treatment modalities can be enthusiastically recommended.
Acknowledgements
Funding: Dr. AM Suskind’s work was funded by the following grant: NIDDK K12 DK83021-07; K12 Urologic Research (KURe) Career Development Program.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wein AJ, Dmochowski RR. Neuromuscular Dysfunction of the Lower Urinary Tract. In: Wein AJ, Kavoussi LR, Novick AC, et al, editors. Campbell-Walsh Urology. 10th ed. Philadelphia: Elsevier Health Sciences, 2011;65:1909-46.
- Tanagho EA, Schmidt RA, Orvis BR. Neural stimulation for control of voiding dysfunction: a preliminary report in 22 patients with serious neuropathic voiding disorders. J Urol 1989;142:340-5. [PubMed]
- Lay AH, Das AK. The role of neuromodulation in patients with neurogenic overactive bladder. Curr Urol Rep 2012;13:343-7. [PubMed]
- van Balken MR, Vergunst H, Bemelmans BL. The use of electrical devices for the treatment of bladder dysfunction: a review of methods. J Urol 2004;172:846-51. [PubMed]
- Kurpad R, Kennelly MJ. The evaluation and management of refractory neurogenic overactive bladder. Curr Urol Rep 2014;15:444. [PubMed]
- Chancellor MB, Chartier-Kastler EJ. Principles of Sacral Nerve Stimulation (SNS) for the Treatment of Bladder and Urethral Sphincter Dysfunctions. Neuromodulation 2000;3:16-26. [PubMed]
- Kessler TM, La Framboise D, Trelle S, et al. Sacral neuromodulation for neurogenic lower urinary tract dysfunction: systematic review and meta-analysis. Eur Urol 2010;58:865-74. [PubMed]
- Dasgupta R, Critchley HD, Dolan RJ, et al. Changes in brain activity following sacral neuromodulation for urinary retention. J Urol 2005;174:2268-72. [PubMed]
- Blok BF, Groen J, Bosch JL, et al. Different brain effects during chronic and acute sacral neuromodulation in urge incontinent patients with implanted neurostimulators. BJU Int 2006;98:1238-43. [PubMed]
- Bemelmans BL, Mundy AR, Craggs MD. Neuromodulation by implant for treating lower urinary tract symptoms and dysfunction. Eur Urol 1999;36:81-91. [PubMed]
- Knüpfer SC, Liechti MD, Mordasini L, et al. Protocol for a randomized, placebo-controlled, double-blind clinical trial investigating sacral neuromodulation for neurogenic lower urinary tract dysfunction. BMC Urol 2014;14:65. [PubMed]
- Brazzelli M, Murray A, Fraser C. Efficacy and safety of sacral nerve stimulation for urinary urge incontinence: a systematic review. J Urol 2006;175:835-41. [PubMed]
- Peters KM, Kandagatla P, Killinger KA, et al. Clinical outcomes of sacral neuromodulation in patients with neurologic conditions. Urology 2013;81:738-43. [PubMed]
- Chaabane W, Guillotreau J, Castel-Lacanal E, et al. Sacral neuromodulation for treating neurogenic bladder dysfunction: clinical and urodynamic study. Neurourol Urodyn 2011;30:547-50. [PubMed]
- Wöllner J, Krebs J, Pannek J. Sacral neuromodulation in patients with neurogenic lower urinary tract dysfunction. Spinal Cord 2015. [Epub ahead of print]. [PubMed]
- Chartier-Kastler EJ, Denys P, Chancellor MB, et al. Urodynamic monitoring during percutaneous sacral nerve neurostimulation in patients with neurogenic detrusor hyperreflexia. Neurourol Urodyn 2001;20:61-71. [PubMed]
- Minardi D, Muzzonigro G. Sacral neuromodulation in patients with multiple sclerosis. World J Urol 2012;30:123-8. [PubMed]
- Marinkovic SP, Gillen LM. Sacral neuromodulation for multiple sclerosis patients with urinary retention and clean intermittent catheterization. Int Urogynecol J 2010;21:223-8. [PubMed]
- Puccini F, Bhide A, Elneil S, et al. Sacral neuromodulation: an effective treatment for lower urinary tract symptoms in multiple sclerosis. Int Urogynecol J 2015. [Epub ahead of print].
- Lombardi G, Musco S, Celso M, et al. Sacral neuromodulation for neurogenic non-obstructive urinary retention in incomplete spinal cord patients: a ten-year follow-up single-centre experience. Spinal Cord 2014;52:241-5. [PubMed]
- Chen G, Liao L. Sacral neuromodulation for neurogenic bladder and bowel dysfunction with multiple symptoms secondary to spinal cord disease. Spinal Cord 2014. [Epub ahead of print]. [PubMed]
- Sievert KD, Amend B, Gakis G, et al. Early sacral neuromodulation prevents urinary incontinence after complete spinal cord injury. Ann Neurol 2010;67:74-84. [PubMed]
- Arlen AM, Powell CR, Kreder KJ. Sacral neuromodulation for refractory urge incontinence is less effective following spinal surgery. ScientificWorldJournal 2011;11:142-6. [PubMed]
- Roth TM. Sacral neuromodulation and lower urinary tract dysfunction in cerebral palsy. Int Urogynecol J Pelvic Floor Dysfunct 2007;18:567-9. [PubMed]
- Marinkovic SP. Sacral neuromodulation is an effective option for non-obstructive urinary retention in men with cerebral palsy. Int J Urol 2014;21:430-1. [PubMed]
- Amundsen CL, Romero AA, Jamison MG, et al. Sacral neuromodulation for intractable urge incontinence: are there factors associated with cure? Urology. 2005;66:746-50. [PubMed]
- Elkelini MS, Hassouna MM. Safety of MRI at 1.5Tesla in patients with implanted sacral nerve neurostimulator. Eur Urol 2006;50:311-6. [PubMed]
- Bartley J, Gilleran J, Peters K. Neuromodulation for overactive bladder. Nat Rev Urol 2013;10:513-21. [PubMed]
- Lombardi G, Nelli F, Mencarini M, et al. Clinical concomitant benefits on pelvic floor dysfunctions after sacral neuromodulation in patients with incomplete spinal cord injury. Spinal Cord 2011;49:629-36. [PubMed]
- Zecca C, Digesu GA, Robshaw P, et al. Motor and sensory responses after percutaneous tibial nerve stimulation in multiple sclerosis patients with lower urinary tract symptoms treated in daily practice. Eur J Neurol 2014;21:506-11. [PubMed]
- Govier FE, Litwiller S, Nitti V, et al. Percutaneous afferent neuromodulation for the refractory overactive bladder: results of a multicenter study. J Urol 2001;165:1193-8. [PubMed]
- Zecca C, Digesu GA, Robshaw P, et al. Maintenance percutaneous posterior nerve stimulation for refractory lower urinary tract symptoms in patients with multiple sclerosis: an open label, multicenter, prospective study. J Urol 2014;191:697-702. [PubMed]
- Gaziev G, Topazio L, Iacovelli V, et al. Percutaneous Tibial Nerve Stimulation (PTNS) efficacy in the treatment of lower urinary tract dysfunctions: a systematic review. BMC Urol 2013;13:61. [PubMed]
- Amarenco G, Ismael SS, Even-Schneider A, et al. Urodynamic effect of acute transcutaneous posterior tibial nerve stimulation in overactive bladder. J Urol 2003;169:2210-5. [PubMed]
- Gobbi C, Digesu GA, Khullar V, et al. Percutaneous posterior tibial nerve stimulation as an effective treatment of refractory lower urinary tract symptoms in patients with multiple sclerosis: preliminary data from a multicentre, prospective, open label trial. Mult Scler 2011;17:1514-9. [PubMed]
- de Sèze M, Raibaut P, Gallien P, et al. Transcutaneous posterior tibial nerve stimulation for treatment of the overactive bladder syndrome in multiple sclerosis: results of a multicenter prospective study. Neurourol Urodyn 2011;30:306-11. [PubMed]
- Kabay S, Kabay SC, Yucel M, et al. The clinical and urodynamic results of a 3-month percutaneous posterior tibial nerve stimulation treatment in patients with multiple sclerosis-related neurogenic bladder dysfunction. Neurourol Urodyn 2009;28:964-8. [PubMed]
- Fjorback MV, van Rey FS, van der Pal F, et al. Acute urodynamic effects of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with MS. Eur Urol 2007;51:464-70; discussion 471-2. [PubMed]
- Ohannessian A, Kaboré FA, Agostini A, et al. Transcutaneous tibial nerve stimulation in the overactive bladder syndrome in patients with Parkinson's syndromes. Prog Urol 2013;23:936-9. [PubMed]
- Krivoborodov GG, Gekht AB, Korshunova ES. Tibial neuromodulation in the treatment of neurogenic detrusor hyperactivity in patients with Parkinson's disease. Urologiia 2006.3-6. [PubMed]
- Kabay SC, Kabay S, Yucel M, et al. Acute urodynamic effects of percutaneous posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with Parkinson's disease. Neurourol Urodyn 2009;28:62-7. [PubMed]
- Monteiro ÉS, de Carvalho LB, Fukujima MM, et al. Electrical stimulation of the posterior tibialis nerve improves symptoms of poststroke neurogenic overactive bladder in men: a randomized controlled trial. Urology 2014;84:509-14. [PubMed]
- Chen G, Liao L, Li Y. The possible role of percutaneous tibial nerve stimulation using adhesive skin surface electrodes in patients with neurogenic detrusor overactivity secondary to spinal cord injury. Int Urol Nephrol 2015;47:451-5. [PubMed]
- Urgent® PC out-patient treatment for bladder and bowel control. Accessed September 14, 2015. Available online: http://www.cogentixmedical.com/patients/products/urgent-pc


