Long-term complications following bladder augmentations in patients with spina bifida: bladder calculi, perforation of the augmented bladder and upper tract deterioration
IntroductionOther Section
Augmentation cystoplasty was originally developed in the 1950’s for the treatment of contracted end stage bladders secondary to tuberculosis and schistosomiasis. The use of this procedure was, however, significantly limited until the advent of clean intermittent catheterization in the early 1970’s. Although waxing and waning in its popularity, augmentation cystoplasty, has remained a part of the urologist’s surgical armamentarium over the last five decades. Indeed, this procedure is still a mainstay in pediatric reconstructive urology where it is used to treat recalcitrant urinary incontinence, in up to 25% of patients with spina bifida and bladder exstrophy (1). In this paper, we desire to review our experience with bladder augmentation in patients with spina bifida. Long-term morbidity within the spina bifida population is affected by a multitude of factors, including: the physiologic activity of the enteric augmentation, the frequent use of bladder outlet procedures and the patient’s mental and/or physical handicaps that may impact self-care. This paper will specifically focus on three major complications: bladder calculi, the most frequent complication found following bladder augmentation, perforation of the augmentation, its most lethal complication and finally we will address loss of renal function as a direct result of our surgical reconstructive procedures.
When evaluating the long-term morbidity of bladder augmentations in spina bifida patients one must remember it is only within the recent decades, that the patients with spina bifida are living longer lives. Indeed, it was not until 2000, that we could finally report that a child born with spina bifida had a 50% chance of survival to 35 years of age (2,3). Regarding long-term follow-up of spina bifida patients we have had a rather unique honor in being able to participate in the transitional care of spina bifida patients from infancy through adulthood, with >300 pts adult pts seen routinely at either yearly or biennial follow-up. In addition, we have managed innumerable patients presenting for second opinions and/or being followed and treated at irregular intervals for management of their urologic complications. This rather unusual experience has resulted in our ability to give some exceptional insight into the long-term management of these patients. We would, however, caution anyone evaluating our outcomes that we serve as a tertiary referral center for individuals with neuropathic urologic complications; our observations could have been greatly affected by referral patterns. To underscore this point we would point out that approximately 50% of our patients were referred to us for either transitional management as they turned into adults or management of complications of their prior surgical interventions (1,4).
Complications of bladder augmentationOther Section
Bladder calculi
Introduction
We began our routine follow-up of patients with a bladder augmentation in 1986. At that time, all of the augments we followed had been performed with either the standard cupped ileal, detubularized ascending colon or a sigmoid bladder augmentation, with catheterization from either a continent abdominal stoma or via the urethra. Routine postoperative management was to irrigate all enteric augmentations with 60 mL of saline daily. Literature from that era suggested that slightly fewer than 50% of the patients developed stones over a 10-year follow-up interval, with approximately 50% of the patients having recurrence of bladder calculi within 5 years (5-7). The highest rate of stone recurrence being related to the presence of an abdominal stoma, with little to no variation in stone recurrence rate based on endoscopic (fragmentation of the stone) versus open (intact) removal (5,6,8-10). Stone formation was presumed to be predominantly related to the retention of mucous within the augmented bladder, with the routine use of bladder irrigation or the use of mucolytic agents believed to significantly reduce the rate of stone formation (5,10-12). The impact of metabolic abnormalities was, however, thought to play a major role in individuals with recurrent bladder calculi with metabolic evaluations revealing hypocitraturia in virtually all individuals with recurrent bladder calculi (13-15). In addition to these ubiquitous findings, low 24-hour urinary volumes (<1,600 mL), hypercalciura and hyperoxaluria were found in one third (13-15).
Materials and methods
All data included and protocols used for patient evaluation within this paper have been approved by the Mayo IRB review, protocol number 07-003450-07. To evaluate the effect of daily bladder irrigations in the prevention of recurrent bladder calculi, we took 75 consecutive patients with spina bifida that presented with bladder calculi following a bladder augmentation between 1986 and 2001. To be included in this study all individuals had to be catheterizing via an abdominal stoma and had an enteric bladder augmentation (48 ileal and 27 colon). They had to agree to maintain a minimum of a yearly follow-up interval for a 10-year period. Prior to placing them on a study protocol all patients underwent removal of existing bladder calculi, by either endoscopic removal with fragmentation (stone size <2 cm in diameter and/or ≤5 in number) or open removal of intact bladder calculi (stone size >2 cm in diameter and/or >5 in number). Patients were maintained on antibiotic prophylaxis for 3 months post stone removal. The prophylactic antibiotic used was based on bacterial sensitivity obtained from culturing fragments of the stone removed. A plain film of the kidneys, ureter and bladder (KUB) and renal bladder ultrasound were obtained at 1 month and 3 months following stone extraction to verify absence of fragments and or missed calculi following treatment. All patients were maintained on bladder irrigations with 60 mL of normal saline during this 3-month time span. If the patient was determined to be stone free at 3 months post stone removal, the patients were divided into the ileal and colonic bladder augmentation subgroups and were then randomly assigned to one of three protocols; irrigation with daily saline wash outs with volumes of either 60 mL (25 pts), 120 mL (25 pts) and 240 mL (25 pts) of saline daily. Non-compliance with bladder irrigation protocols was defined as a 3-month or greater time span when the patient missed more than one episode of bladder irrigation per week. Follow-up was performed at yearly intervals with routine assessment of serum electrolytes, creatinine, renal function tests, urine cultures, renal and bladder ultrasound and a KUB. Cystoscopic evaluations were performed in individuals with a history of ≥4 symptomatic urinary tract infections per year, gross hematuria or microscopic hematuria of >50 RBC/HPF, a history of persistent abdominal or pelvic pain or radiographic abnormalities suggestive of a bladder calculi or obstruction. The augmented bladder was noted to be colonized with bacteria if a routine yearly bacterial culture grew out a bacteria at >105 colonies per mL of urine and the patient was asymptomatic. Symptomatic urinary tract infections (UTIs) were defined as a positive urine culture at >105 colonies per mL occurring in conjunction with either a temperature ≥38.5 °C, chronic malaise and fatigue, new onset of flank, pelvic or bladder pain or persistently grossly purulent/foul smelling urine lasting >72 hours. Individuals with ≥4 symptomatic UTI per year were placed on oral antibiotic prophylaxis. The presence or recurrence of a bladder calculus was confirmed by both radiographic and endoscopic findings. Statistical evaluations used Kaplan Meir Curves, chi-square analysis or two tailed t-tests where appropriate, P values <0.05 were considered significant.
Results
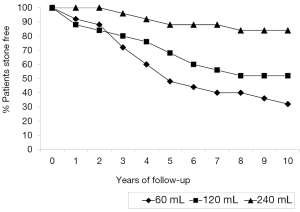
Non-compliance with medical directives regarding bladder irrigation was not significantly different between any of the three treatment groups, 60 mL-12% (3/25); 120 mL-16% (4/25) and 240 mL-20% (5/25), P=0.44. Fifty percent (6/12) of the individuals who were non-compliant with irrigation protocols developed renal calculi. Noncompliance with irrigation was not associated with recurrent stones in patients irrigating with 60 mL-66% (1/3) vs. 68% (15/22), P=0.95 or in patients irrigating with 120 mL-50% (2/4) vs. 48% (10/21), P=0.93, However, noncompliance with bladder irrigations with 250 mL was associated with recurrent bladder calculi, 50% (2/4) vs. 11% (2/19), P=0.429. Figure 1 demonstrates the recurrence of bladder calculi based upon volume of bladder irrigations. Notably the volume of daily bladder irrigant of 250 mL significantly reduced the incidence of recurrent stone formation compared to bladder irrigations of either 60 mL (P<0.0002) or 120 mL (P=0.0152) by the seventh year following the initial stone extraction. The use of high volume 240 mL bladder irrigations was also found to significantly decrease the incidence of bacterial colonization of the bladder as determined by the yearly surveillance urine cultures. Specifically, 45% (113/250) of the routine yearly urine cultures were positive in individuals irrigating with 240 mL, 62% (155/250) in individuals irrigating with 120 mL (P<0.001) and 84% (210/250) in the individuals irrigating with 60 mL (P=0.00039). High volume irrigations also decreased the incidence of symptomatic UTI over a 10-year time span: 47 in the 240 mL irrigation group compared to 100 in the 120 mL group (P<0.0001) and 127 in the 60 mL group (P<0.0001).
Evaluation and treatment of recurrent stone formers while on irrigation protocols
A total of 44% (33/75) of our patients developed recurrent bladder calculi while on an irrigation protocol. All of these individuals were subsequently placed on high volume bladder irrigations with 240 mL and assessed for the presence of urinary metabolic abnormalities. Twenty-four-hour urines for volume, creatinine, calcium, oxalate and citrate were obtained. These evaluations revealed metabolic abnormalities to be present in 91% (30/33). Hypocitraturia (<320 mg/24 hours) was found in 75% (25/33) patients. Mixed metabolic anomalies with low urine output (≤1,600 mL), hypercalciuria (>300 mg/24 hours) and hypocitraturia were found in 15% (5/33). In the 25 pts with isolated hypocitraturia as their only metabolic defect, 12 patients were placed on irrigation of 250 mL of saline daily and 13 were placed on 250 mL of saline bladder irrigations daily along with potassium citrate supplementation. The amount of citrate administered varied, with dosage being dependent upon normalization of urinary citrate levels as measured by a 24-hour urine collection. In the eight remaining patients (three with no metabolic abnormalities and five with combined defects) we placed the patients on irrigation of the bladder with 250 mL of saline daily and instilled 30 mL of 20% acetylcysteine into the bladder at night (16,17). All patients were followed for a 5-year time period. Non-compliances with medical directives among the recurrent stone formers were similar between all treatment groups: 8% (1/12) in patients irrigating with 250 mL, 15% (2/13) in individuals on both citrate and irrigation and 12% (1/8) in patients using acetylcysteine and irrigation. P=0.587. Figure 2 represents the comparison in stone free rates between the three interventional strategies, no significant statistical difference was found between any of the treatment methods, with bladder irrigation with 250 mL alone being as successful as citrate replacement therapy or the use of the mucolytic agent acetylcysteine. Stone free rates at 5 years were 67% (8/12) in patients irrigating with 250 mL of saline, 69% (9/13) in patients on citrate therapy and irrigation and 63% (5/8) in patient on acetylcysteine and irrigation, P>0.5.
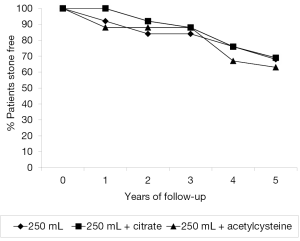
Conclusions
The development of bladder calculi is the most frequent complication found following enteric bladder augmentation, having been reported to occur in up to 50% of the patients undergoing this procedure with a 50% incidence of recurrent stones within 5 years of their first occurrence (5-10). The etiology of the stone formation has been hypothesized to be due to either mucous retention in the bladder serving as a nidus for stone infection, chronic bacterial colonization or underlying metabolic abnormalities caused by loss of the bowel from the gastrointestinal tract and/or its incorporation in to the urinary system. To combat the development or recurrence of bladder calculi, a variety of treatment modalities have been employed; high volume irrigation of the bladder, correction of metabolic abnormalities and/or the use of mucolytic agents (7,11,12,16,17). In the mid 1980’s, we elected to study a select a group of patient’s that had a high probability of developing calculi, specifically, individuals who had already formed previous bladder calculi and were catheterizing via an abdominal stoma. By selecting out high risk patients we believed that we would be able to best determine if sequential increases in bladder irrigant volumes would result in a statistically significant decrease in the development of recurrent bladder calculi. The information we garnered from this study, that is, high volume bladder irrigations of 240 mL or greater performed on a daily interval is associated with both a significant decrease in recurrent bladder calculi (P<0.05) and a decrease in symptomatic urinary tract infections (P<0.0001). These findings resulted in us using daily high volume bladder irrigations (>240 mL) as one of the backbones for postoperative management of these patients for the past decade. It is noteworthy that our findings are supported by a study from Dr. Hensle and associates (10). Although high volume bladder irrigations help decrease the incidence of stone formation, it is far from a panacea with stone recurrence rates approaching 10–15% at 10 years. In individuals with ≥2 recurrent bladder stones we subsequently performed metabolic evaluations to evaluate for underlying abnormalities. Similar to prior published reports, we found a high incidence of metabolic anomalies (91%; 30/33), within this select patient population (13-15). Although treatment of metabolic abnormalities has been documented to decrease renal calculi, it is unknown if treatment of the metabolic abnormality will decrease the incidence of recurrent bladder calculi (18,19). In view of the multiple factors that could causes recurrent bladder calculi within this population such as, poorly configured augments resulting retained mucous, bacteriuria, patient noncompliance with medical directives, we were unsure if treatment of the metabolic abnormality would indeed prevent recurrent bladder stones or if retention of mucous despite high bladder irrigation was the primary problem. We therefore studied individuals who were catheterizing through abdominal stomas and had been documented to have two or more instances of bladder calculi. In this patient population if a metabolic defect was identified we chose to either; (I) actively treat the metabolic disease and use high bladder volume irrigation, (II) use high bladder volume irrigation plus a mucolytic agent or (III) use high bladder volume irrigation alone. It was our hope that this study would show us the best way to treat patients with recurrent bladder stones that are recalcitrant to high volume bladder irrigation. In this study, we found no benefit in decreasing stone recurrence rates by correction of the underlying metabolic defect, nor by the addition of mucolytic agents, with stone free rates in 5 years almost identical in all groups, ranging from 63–68%. Currently, in our patients that are forming recurrent bladder stones that are recalcitrant to high volume bladder irrigation, (e.g., ≥3 episodes of recurrent bladder stones) we will in addition to the high bladder volume irrigations, initiate the use of 30 mL of either 20% urea or 20% acetylcysteine solution into the bladder at night (12,16,17). We would caution that we have absolutely no clinical documentation that this treatment will substantially decrease the risk of additional stones and is done as an empiric trial.
We believe a word of caution needs to be raised regarding treatment of the underlying metabolic defect in the prevention of recurrent bladder calculi. When our data failed to document that the treatment of hypocitraturia decreased the risk of bladder stones over high volume bladder irrigation alone, we stopped the use of citrate replacement within our patients. Interestingly, long-term follow-up of the patients with hypocitraturia (median 18 years, range, 12–30 years) has revealed calcium oxalate or mixed hydroxyapatite/calcium oxalate nephrolithiasis to develop in 12% (3/25). The development of metabolically derived renal calculi in patients following a bladder augmentation has been described previously (20). Whether continued treatment of this patient population by citrate therapy would have decreased that risk for renal calculi is unknown.
Perforation of the bladder augmentation
Introduction
Spontaneous or traumatic rupture of an augmented bladder is associated with significant morbidity and has a reported mortality incidence of 25% (21,22). The incidence of this life threatening complication varies widely dependent upon the size of the study population and the length of follow-up, with a reported incidence ranging from 2–13% (23-26). Multiple of factors have been found to play a role in the incidence of this complication; including the choice of bowel segment used for the augment, ischemic injury to the augmenting segment, persistent high bladder pressure, chronic or acute bladder over distension, fixed adhesions of the augment to pelvic or abdominal wall, trauma from catheterization, chronic transmural bladder-bowel wall infection, and closure of the bladder neck (22,24,25,27,28). Prior studies have suggested that the median time from augmentation to perforation was 3 years (28). Of note, due to our work in transitional urology with lifelong follow-up of our spina bifida population we have a slightly different finding. The majority of our spontaneous bladder perforations are being found in patients in their late teens, early 20’s and 30’s. Indeed, long-term follow-up of our spina bifida population has revealed the most significant relationship regarding the risk of bladder perforation are none of the factors noted above, but rather, a direct relationship to either substance abuse and/or patient noncompliance with intermittent catheterization.
Materials and methods
From 1986-2009 we screened 203 patients (≥16 years of age) for the presence of alcohol abuse and noncompliance with medical directives. The definition of alcohol abuse was based on the criteria as set forth by the National Institute on Alcohol Abuse and Alcoholism as consumption of two or more drinks per day. Non-compliance with medical directives for intermittent catheterization was defined as the patient admitting to catheterizations being performed 3 or fewer times per day on a routine basis. All patients had a medical history obtained and underwent laboratory evaluations at yearly Intervals. If the medial history confirmed noncompliance with directives and/or if laboratory evaluations documented metabolic abnormalities (i.e., hyperchloremic metabolic acidosis) that corrected with the use of intermittent catheterization at 4-hour diurnal intervals, a diagnosis of non-compliance with medical directives was made. A total of 12% (24/203) of our patients had a history of alcohol abuse with or without concurrent noncompliance with intermittent catheterization, an additional 6% (12/203) were noncompliant with catheterization without associated alcohol abuse. In the 36 patients with a history of alcohol abuse and/or patient noncompliance with catheterization, 22% (8/36) experienced a spontaneous rupture of the bladder. A total of 14 perforations and one death secondary to overwhelming sepsis occurred within these eight patients. Two patients were converted to ileal conduits due to repeated bladder perforations (n=3) and failure to gain sobriety. It is noteworthy that other illicit drug use besides alcohol was present in 11% (4/36) and a diagnosis of intellectual disability was present in 16% (6/36). This is compared to an incidence of spontaneous bladder rupture of 2% (3/67) in patients without a history of alcohol abuse or noncompliance with intermittent catheterization. The median length of follow-up of both groups of was 24 years since the time of the augmentation, range 8–53 years. The relationship between alcohol abuse and noncompliance with intermittent catheterization to bladder rupture was significant, P=0.00595.
Conclusions
Our viewpoint on spontaneous bladder perforation following a bladder augmentation has been significantly biased by a practice heavily based in the transitional and adult care of the spina bifida patient. This experience has resulted in a unique viewpoint that is focused on the need of the patient to be compliant with medical directives and to refrain from risky behavior to prevent some of the major complications associated with this procedure. Specifically, how can a physician assess the ability to be compliant with medical directives and instruct a patient to refrain from risky behavior in 9 years old, the median age for a bladder augmentation within the spina bifida patient population (23,28)? Indeed, augmentations are routinely performed in early childhood and/or in patients with mental impairment, with the physician relying on adult surrogates to either perform or prompt the patient to implement their bladder care. With the onset of the patient’s adolescence and/or maturation of parent-child relationship, the individual’s ability to be compliant with the medical directives may become problematic. Compound this finding with a failure to instruct the patient regarding the need to prevent high-risk behavior, that is, to refrain from excessive alcohol or illicit drug use after a bladder augmentation and we are setting our patients up for failure. Due to our experience we have significantly altered our preoperative assessment and teaching. All patients are currently instructed and then quizzed in the knowledge of the care that is necessary to maintain proper bladder hygiene and instructed in the need to reduce risky behavior. Consideration for proceeding to surgery occurs only after the patient him/herself can repeatedly document they have acquired the foundation of knowledge necessary to maintain their health.
Upper tract deterioration
Introduction
Reconstructive urologic surgery has four major goals: preserve life, maintain or improve renal function, maintain or obtain urinary continence and the desire to maintain or obtain sexual function. Outside of the preservation of life, maintenance of renal function is the second key metric in appraising the morbidity of genitourinary reconstructive procedures. In this regard the surgeon should always balance the ability to provide urinary continence with the risk of losing renal function. In children with spina bifida, upper tract deterioration following continence producing procedures such as placement of an artificial urinary sphincter, urinary slings and/or noncompliance with intermittent catheterization has been known to result in renal injury (29). This portion of the paper looks at our experience with preservation of renal function following the performance of an augmentation cystoplasty when combined with a procedure to increase outlet resistance.
Materials and methods
To assess the risk of upper tract deterioration following a bladder augmentation we have maintained a prospective database since 1986. This database includes patients we have either performed an augmentation cystoplasty on and/or who have been transferred to our transitional care at adulthood. Only patients with spina bifida that had documented normal upper tracts and renal function at the time of their simultaneous bladder augmentation and bladder neck outlet procedure (bladder neck closure, reconstruction or sling) were included. Outside films and medical records were reviewed for their baseline evaluations. Baseline renal architecture was determined to be normal by one of three methods; renal ultrasonography, intravenous pyelography or by CT scan with contrast. The initial renal function was determined by using the baseline serum creatinine and calculating the GFR using the Cockcroft and Gault formula. Once patients were entered into our registry, preservation of renal architecture was annually evaluated with renal ultrasounds and renal clearance determinations. The method of GFR determination has varied through the years. Renal clearance was initially evaluated using creatinine clearance [1986–1993], iothalamate clearance [1994–2010] or estimated by cystatin C determination (2010–date). Suspicion for the development of a renal scar on ultrasonography was confirmed with either a DMSA or contrast enhanced CT scan. Renal insufficiency was defined as a renal clearance value of <60 cc/min/BSA (stage 3–4 chronic renal failure). Clearance was determined as an average of the three most recent clearance values obtained. End stage renal disease (ESRD) was defined as the need to begin dialysis or by the patient receiving a pre-emptive renal transplantation. Renal preservation was defined as the patient maintaining a renal clearance of ≥60 cc/min/BSA and the absence of scars on renal ultrasound. Noncompliance with intermittent catheterization was defined as the patient admitting to catheterizations being performed three or fewer times per day on a routine basis and/or if laboratory evaluations documented metabolic abnormalities (i.e., hyperchloremic metabolic acidosis) that corrected with the use of intermittent catheterization at 4-hour diurnal intervals.
Results
A total of 80 patients were managed by ileocystoplasty and a bladder neck outlet procedure (bladder neck closure, reconstruction or sling); median follow-up was 14 years (range, 8–45 years). All patients had been documented to have vesicoureteral reflux absent by a cystogram prior to surgery. Deterioration of the upper tracts as defined by the new onset of renal scars occurred in 40% (32/80). Development of ≥ stage 3 chronic renal failure occurred within 38% (12/32) of the patients with scarring, 15% (12/80) of the total patient population. Despite a relatively high incidence of renal scarring, the median renal clearance at last follow-up, excluding those with ESRD, was 95 cc/min/BSA (range, 20–130 mL/min/BSA). Prior to the onset of upper tract deterioration, 69% (22/32) of the patients had been noncompliant with intermittent catheterization, 46% (15/32) had a struvite renal calculus associated with episodic pyelonephritis and 34% (11/32) had developed a bladder calculus, six (54%, 6/11) of whom developed new onset of reflux/hydronephrosis related to the calculus. The onset of upper tract deterioration (i.e., new scar formation, hydronephrosis, calculus development, decrease in renal function) was clinically asymptomatic in one third (10/32 pts).
Discussion
It is our opinion that outside of the preservation of life, maintenance of renal function is the key metric to appraise the worth of a genitourinary reconstructive procedure. It is indeed concerning to see that renal scarring and/or loss of a kidney due to nonfunction occurred in 40% (32/80) of our patients undergoing a bladder augmentation with simultaneous bladder outlet procedure. Add to this finding that 38% (12/32) of the patients with scarring developed ≥ stage 3 chronic renal failure during a median follow-up interval of 14 years and the apprehension heightens. The observation that 15% (12/80) of our total patient population had developed stage 3 chronic renal failure (GFR <60 mL/min/BSA) is sobering. Specifically, when compared with healthy populations, patients with a GFR of 41–59 mL/min/BSA have a 20% increase in cardiovascular related death, this risk increases with serially worsening renal failure and will reach up to 59% in patients with a GFR ≤15 mL/min/BSA (30,31). It is therefore incumbent upon the physicians who follow these patients to identify the etiology and frequency of renal insufficiency and delineate ways to prevent its development. Of particular concern is the fact that the vast majority of patients with upper tract deterioration, 69%, developed this problem as a consequence of noncompliance with intermittent catheterization. Even perhaps more disturbing is that they did not manifest their non-compliance until their late teens, twenties and even thirties, decades after their surgical procedure. It is also noteworthy that one third of the patients with upper tract deterioration were clinically asymptomatic. That is, their medical history revealed no history of recurrent urinary tract infections, chronic fatigue or lethargy, hematuria, flank, abdominal or pelvic pain. Documentation of renal deterioration was found only at the time of routine follow-up evaluation. These findings strongly suggest that patients who have had a bladder augmentation performed warrant routine interval life-long follow-up (4,32). Our current routine is to see all patients who have had a bladder augmentation on an annual basis. We routinely obtain a renal bladder ultrasound, KUB, serum electrolytes, creatinine, cystatin C and urine culture. We selectively employ endoscopic evaluations in patients with either an abnormal radiographic study or a positive medical history for four or more symptomatic UTIs per year, history of gross hematuria, a urinalysis revealing >50 RBC/HPF, chronic perineal, pelvic or bladder pain. We also employ endoscopy in all patients with a colonic augment at age 50 years or greater consistent with recommendations for colonoscopy.
ConclusionsOther Section
This paper is a review heavily based on the prolonged follow-up of spina bifida patients following their transition to adult care. It documents the need for high volume bladder irrigations to both prevent the most common complication following bladder augmentation, which is, the development of bladder calculi and to reduce the incidence of symptomatic urinary tract infections. It provides a unique perspective regarding the impact of substance abuse and patient non-compliance with medical directives as both the most common cause for spontaneous bladder rupture following augmentation cystoplasty and for deterioration of the upper tracts. These findings should cause the surgeon to reflect on his/her assessment of a patient prior to performing a bladder augmentation procedure. Indeed, true surgical wisdom does not lie in knowing how to do an operation, but rather in knowing the correct patient and time to employ it.
AcknowledgementsOther Section
None.
FootnoteOther Section
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethics and research committee IRB number 07-00350, informed consent for the use of medical information for research and quality control purposes is signed (approved) by all patients undergoing care at the Mayo Clinic.
ReferencesOther Section
- Higuchi TT, Granberg CF, Fox JA, et al. Augmentation cystoplasty and risk of neoplasia: fact, fiction and controversy. J Urol 2010;184:2492-6. [PubMed]
- Woodhouse CR. Progress in the management of children born with spina bifida. Eur Urol 2006;49:777-8. [PubMed]
- Hunt GM. Open spina bifida: outcome for a complete cohort treated unselectively and followed into adulthood. Dev Med Child Neurol 1990;32:108-18. [PubMed]
- Husmann DA, Fox JA, Higuchi TT. Malignancy following bladder augmenation: recommendations for long-term follow-up and cancer screening. AUA Update Ser 2011;30:222-7.
- Austin JC. Long-term risks of bladder augmentation in pediatric patients. Curr Opin Urol 2008;18:408-12. [PubMed]
- Roberts WW, Gearhart JP, Mathews RI. Time to recurrent stone formation in patients with bladder or continent reservoir reconstruction: fragmentation versus intact extraction. J Urol 2004;172:1706-8; discussion 1709.
- Szymanski KM, Misseri R, Whittam B, et al. Cutting for stone in augmented bladders-what is the risk of recurrence and is it impacted by treatment modality? J Urol 2014;191:1375-80. [PubMed]
- Metcalfe PD, Cain MP, Kaefer M, et al. What is the need for additional bladder surgery after bladder augmentation in childhood? J Urol 2006;176:1801-5; discussion 1805.
- Barroso U, Jednak R, Fleming P, et al. Bladder calculi in children who perform clean intermittent catheterization. BJU Int 2000;85:879-84. [PubMed]
- Hensle TW, Bingham J, Lam J, et al. Preventing reservoir calculi after augmentation cystoplasty and continent urinary diversion: the influence of an irrigation protocol. BJU Int 2004;93:585-7. [PubMed]
- Khoury AE, Salomon M, Doche R, et al. Stone formation after augmentation cystoplasty: the role of intestinal mucus. J Urol 1997;158:1133-7. [PubMed]
- DeFoor W, Minevich E, Reddy P, et al. Bladder calculi after augmentation cystoplasty: risk factors and prevention strategies. J Urol 2004;172:1964-6. [PubMed]
- Robertson WG, Woodhouse CR. Metabolic factors in the causation of urinary tract stones in patients with enterocystoplasties. Urol Res 2006;34:231-8. [PubMed]
- Woodhouse CR, Robertson WG. Urolithiasis in enterocystoplasties. World J Urol 2004;22:215-21. [PubMed]
- Hamid R, Robertson WG, Woodhouse CR. Comparison of biochemistry and diet in patients with enterocystoplasty who do and do not form stones. BJU Int 2008;101:1427-32. [PubMed]
- Benderev TV. Acetylcysteine for urinary tract mucolysis. J Urol 1988;139:353-4. [PubMed]
- Covert WM, Westin SN, Soliman PT, et al. The role of mucoregulatory agents after continence-preserving urinary diversion surgery. Am J Health Syst Pharm 2012;69:483-6. [PubMed]
- Kadlec AO, Turk TM. Update on the evaluation of repeated stone formers. Curr Urol Rep 2013;14:549-56. [PubMed]
- Marchini GS, Ortiz-Alvarado O, Miyaoka R, et al. Patient-centered medical therapy for nephrolithiasis. Urology 2013;81:511-6. [PubMed]
- Matlaga BR, Kim SC, Watkins SL, et al. Changing composition of renal calculi in patients with neurogenic bladder. J Urol 2006;175:1716-9; discussion 1719.
- Blok BF, Al Zahrani A, Capolicchio JP, et al. Post-augmentation bladder perforation during urodynamic investigation. Neurourol Urodyn 2007;26:540-2. [PubMed]
- Elder JS, Snyder HM, Hulbert WC, et al. Perforation of the augmented bladder in patients undergoing clean intermittent catheterization. J Urol 1988;140:1159-62. [PubMed]
- Fox JA, Husmann DA. Continent urinary diversion in childhood: complications of alcohol abuse developing in adulthood. J Urol 2010;183:2342-6. [PubMed]
- DeFoor W, Tackett L, Minevich E, et al. Risk factors for spontaneous bladder perforation after augmentation cystoplasty. Urology 2003;62:737-41. [PubMed]
- Shekarriz B, Upadhyay J, Demirbilek S, et al. Surgical complications of bladder augmentation: comparison between various enterocystoplasties in 133 patients. Urology 2000;55:123-8. [PubMed]
- Krishna A, Gough DC, Fishwick J, et al. Ileocystoplasty in children: assessing safety and success. Eur Urol 1995;27:62-6. [PubMed]
- Ehdaie B, Mason MD, Gray M, et al. Bladder perforation in augmentation cystoplasty during urodynamic investigation: a case report and review of the literature. J Pediatr Urol 2013;9:e102-6. [PubMed]
- Metcalfe PD, Casale AJ, Kaefer MA, et al. Spontaneous bladder perforations: a report of 500 augmentations in children and analysis of risk. J Urol 2006;175:1466-70; discussion 1470-1. [PubMed]
- Levesque PE, Bauer SB, Atala A, et al. Ten-year experience with the artificial urinary sphincter in children. J Urol 1996;156:625-8. [PubMed]
- Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305. [PubMed]
- Go AS, Yang J, Ackerson LM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation 2006;113:2713-23. [PubMed]
- Higuchi TT, Fox JA, Husmann DA. Annual endoscopy and urine cytology for the surveillance of bladder tumors after enterocystoplasty for congenital bladder anomalies. J Urol 2011;186:1791-5. [PubMed]