Urodynamic and physiologic patterns associated with the common causes of neurogenic bladder in adults
Introduction
Complete evaluation of the patient with neurologic bladder dysfunction is important for goal oriented treatments. Although the causes of the dysfunction and their subsequent effects can be vast, with appropriate evaluation, the clinician can break these down into basic and treatable factors. These factors include function or dysfunction of storage, detrusor activity, and pelvic floor response. Urodynamics (UDS) may be considered as the gold standard in helping to break down complex and multifactorial voiding dysfunction into manageable headings; these include protecting the upper tracts, limiting urinary tract infections (UTI) via avoiding urinary stasis, and maintaining quality of life. Experts believe all neurogenic patients should undergo a baseline UDS study and as needed for any clinical changes or new symptoms (1,2). Herein we outline the basic elements seen in patients with neurogenic bladder on UDS, and augment these with examples of lesions, for any cause, at clinically relevant levels of the neurologic pathway.
Non-invasive UDS
Non-invasive UDS are inexpensive, readily available, have low morbidity and are a great option for an initial evaluation. Post-void residuals (PVR) and uroflowmetry are the keystones of this evaluation. Using ultrasound the bladder can be scanned after a void to estimate a residual volume. Many experts believe that a PVR of <100–200 indicates acceptable emptying to prevent infection and normal detrusor function (3). Uroflowmetry is the measurement of voided urine over the course of time and can be calculated and compared to established nomograms (4). For abnormal findings these studies have their limitations in isolating elements of the voiding cycle that may be malfunctioning such as detrusor vs. pelvic floor dysfunction or outlet issues. The combination of these two studies plays an important role for initial evaluation prior to invasive UDS evaluation and after treatment as a low cost and well tolerated set of tools.
Invasive UDS
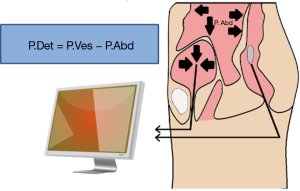
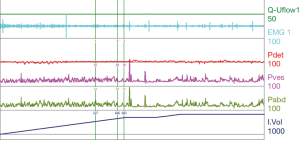
For the patient with neurologic voiding dysfunction, invasive UDS may be the most powerful and helpful assessment tool set. The basis of an invasive urodynamic study is obtaining pressure readings and myogenic readings of the pelvic floor with the use of specifically placed catheters and electrodes. This information helps give a graphical interpretation of pressures within and produced by the bladder, abdominal cavity, and the pelvic floor musculature (Figure 1). A pressure sensing catheter is placed in the bladder and the rectum. These values, along with the electromyography (EMG), can help give a physiologic description of the dynamics in the phases of filling, storage, and voiding.
Components of the urodynamic evaluation
Filling/storage
Patients with neurologic bladder dysfunction may have a wide array of storage capacity abnormalities ranging from very small volumes to large distended bladders. Typical adult bladders have a capacity of 300–600 cc (5). Decreased volumes can cause frequency and make incontinence worse whereas large volumes can contribute to urinary stasis and increase the risk of UTI.
Compliance
Compliance is calculated by the change in volume divided by the change in pressure (Table 1). This represents the bladder’s ability to maintain storage at low pressures as the bladder fills. These measurements rely on accurate recording of the rectal pressure/abdominal pressure (Pabd) and intravesical pressure (Pves), which together allow one to calculate the detrusor pressure (Pdet), with Pves – Pabd = Pdet. A physiologically normal bladder has a relatively constant low bladder pressure throughout the filling cycle, resulting in a high compliance. A normal compliance can reach infinity and an abnormal compliance is variably defined as <40 or <20 cc/cmH2O. Decreased compliance, or sustained Pdet greater than 40 cmH2O, can put the upper tracts at risk for elevated pressures resulting in renal atrophy and infection risk.
Bladder sensation (sensory)
Normal bladder sensation can be judged by three defined points noted during the filling cycle and evaluated in relation to the bladder volume (Table 1). In the patient with neurologic bladder dysfunction bladder sensation can be absent, decreased, or heightened. Noting the following, with exact definitions, during the filling cycle can be useful in evaluation:
- First sensation of bladder filling: is the feeling the patient has, during filling cystometry, when he/she first becomes aware of the bladder filling;
- First desire to void: is defined as the feeling, during filling cystometry, that would lead the patient to pass urine at the next convenient moment, but voiding can be delayed if necessary;
- Strong desire to void: a persistent desire to void without fear of leakage (6).
Detrusor function (motor)
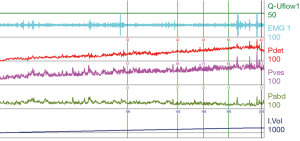
Function of the detrusor muscle is comprised of two components, its ability to passively allow filling (compliance) and its ability to contract upon voluntary micturition. This is measured in UDS by Pdet and voided volumes. Decreased relaxation during filling can contribute to storage issues as well as overactivity. Detrusor overactivity (DO) is defined as involuntary detrusor contractions, of variable duration and amplitude, during filling that are unable to be suppressed by the patient (Table 1) (6). DO can contribute to urgency and frequency, incontinence at low storage volumes, and elevated pressures if not coordinated with sphincteric relaxation during micturition (see below). Poor contraction at large volumes can decrease or eliminate the patient’s ability to void to completion thus contributing to an ever increasing volume, and possible increased risk of urinary retention and upper tract deterioration over time.
Sphincter activity
This represents the external urinary sphincter’s ability to maintain contraction and relaxation at appropriate times throughout the filling and voiding cycle (Table 1). This is measured in UDS by the EMG tracing. A normal sphincter mechanism shows activity as manifested by increased recruitment during the filling cycle, increasing as the bladder volume increases to maintain continence. Failure of the sphincter to maintain contraction leads to incontinence and low pressures/volumes of leaking. During the voiding cycle, or during a coordinated detrusor contraction, one normally sees silence of the sphincteric myogenic activity or relaxation (7).
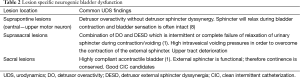
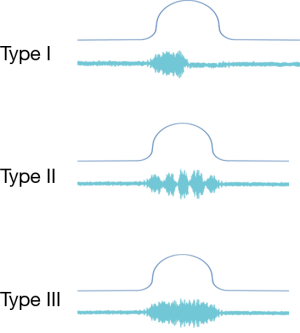
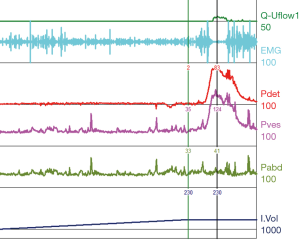
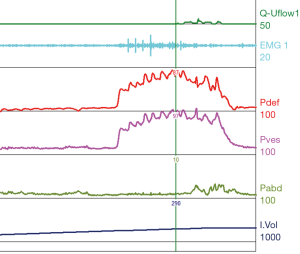
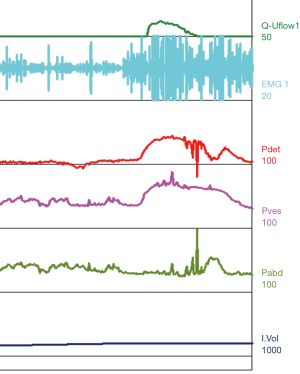
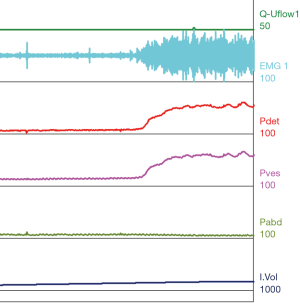
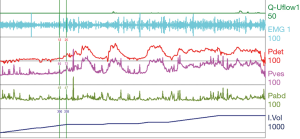
A common finding in patients with neurologic bladder dysfunction is discoordination of the sphincter during contraction [detrusor sphincter dyssynergia (DSD)] (Table 2) (9). By setting up a situation where the bladder contracts against a non-coordinated closed sphincter, this may over time, create high Pves putting the upper tracts at risk for deterioration (10). There are three main types of DSD (Figure 2). In type I there is a synchronous increase in the Pdet and sphincteric tone until the peak of detrusor function at which point the sphincteric tone relaxes and unobstructed voiding occurs. Type II DSD is characterized by sporadic contractions of the sphincter throughout the detrusor function. Type III DSD is similar to type I in which the sphincteric tone increases throughout the detrusor function however, contrary to type I, relaxation is never observed and results in obstructed voiding. Patients with type III DSD are at highest risk for upper tract deterioration due to elevated pressures during micturition and decreased voided volumes.
Full table
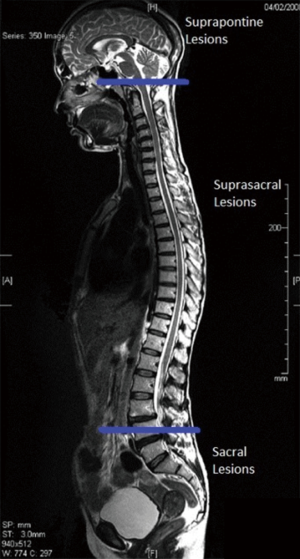
Patterns of voiding dysfunction with fixed lesions
Urinary dysfunction is common with fixed lesion spinal cord injury with approximately 81% of patients having some element of voiding dysfunction (11). The degree of UDS findings and voiding dysfunction can fall into three categories based the level of the neurologic lesion; suprapontine (central—upper motor neuron), suprasacral, and sacral lesions (Figure 3). Based on these levels capacity, compliance, contractility, and pelvic sphincteric function have predictable patterns on UDS, all of which will help clinicians in the clinical management of the patient (Table 2). In patients with a single level of spinal cord injury the level of injury and the type of voiding dysfunction has a stronger correlation. Patients with combined suprasacral and sacral injuries can have relatively unpredictable urodynamic findings (8). Management of the urinary tract in patients with spinal cord injury must be based on urodynamic findings rather than inferences from the neurologic evaluation. In reality many disease processes will have some elements that do not fall within the simplified groupings outlined for patients with fixed spinal cord defects above. However, again simplifying these complex lesions into three clinically relevant categories can be a useful tool for the clinician in driving realistic and beneficial treatments (1).
Neurologic bladder dysfunction in specific neurological disorders
Neurologic disorders often do not fit as neatly into predicted patterns of voiding dysfunction as do fixed spinal cord lesions. However there are some predictable findings on UDS which are experienced with increased frequency and will be outlined in the paragraphs below and Table 3.

Full table
Multiple sclerosis (MS)
MS and other demyelinating disorders can have highly variable clinical presentations based on the amount of disease burden. Most commonly UDS tracings will show some element of DO, when combined with decreased capacity and compliance, storage issues can be in the forefront. Each patient should be evaluated for safety of the upper tracts as DSD is common.
Parkinson’s disease (PD)
PD patients generally present with overactive bladder symptoms, neurologic DO on UDS, and urge incontinence. These symptoms are generally uniform early in the disease process for all patients. A subset of the population will have symptoms progress to global hypoactivity with decreased capacity, passive filling resulting in decreased compliance and decreased sphincteric function. Retention generally is not a clinical problem given worsening sphincter tone.
Spina bifida (SB)
Clinical and UDS findings with SB are highly variable. Increases and decreases in capacity, compliance, detrusor activity and sphincteric function can be observed throughout the vast gambit of SB presentations. As such, frequent and repeat UDS with any clinical change has become the corner stone of care. One finding found with increased frequency in the SB population is DSD, often severe, and if gone unidentified can put the patient’s upper tracts at significant risk for deterioration.
Diabetes mellitus (DM) and peripheral neuropathy (PN)
DM and its associated PN, or PN from other causes, can have significant clinical impacts on voiding dysfunction. Clinical symptoms worsen with disease progression and generally include declining detrusor function and over time results in larger bladder volumes, increased compliance. Depending on the degree of decrease in sphincteric function clinical presentation can present at urinary retention, more commonly in men with higher outlet resistance, to incontinence with high residuals in females.
Cerebral vascular accident (CVA)
Clinical presentation can be highly variable with variability in location of the upper motor neurons affected. However, seen with some frequency are detrusor underactivity and incontinence problems with decreased sphincteric function. As in DM and PN the degree of residual volumes can depend on the severity of decreased sphincteric function (1,9,12,13).
Urodynamic tracings and examples of key findings
For reference and clinical comparison outlined below are figures which include urodynamic tracings of normal (Figure 4), three types of DSD (Figures 5-7), several categories of neurogenic voiding dysfunction including decreased compliance and detrusor over activity (Figures 8-10).
Summary
The evaluation of a patient with neurogenic bladder dysfunction with UDS provides a powerful tool in the identification of the disease specific manifestation of voiding dysfunction and can create a framework for goal directed management. A full UDS evaluation contributes a baseline with which disease progression can be monitored, and periodic assessment of risk for upper tract deterioration, and identification of clinically beneficial interventions may be performed intermittently as the clinical situation dictates. Patterned findings often correlate to lesion level/location or disease process. Pattern recognition and understanding of UDS can help lead to quality of life improvements and optimal management for the patient with neurogenic bladder dysfunction.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Goldmark E, Niver B, Ginsberg DA. Neurogenic bladder: from diagnosis to management. Curr Urol Rep 2014;15:448. [PubMed]
- Veenboer PW, Bosch JL, Rosier PF, et al. Cross-sectional study of determinants of upper and lower urinary tract outcomes in adults with spinal dysraphism--new recommendations for urodynamic followup guidelines? J Urol 2014;192:477-82. [PubMed]
- Vodušek DB, Boller F. Introduction. Handb Clin Neurol 2015;130:3-7. [PubMed]
- Oelke M, Rademakers KL, van Koeveringe GA, et al. Unravelling detrusor underactivity: Development of a bladder outlet resistance-bladder contractility nomogram for adult male patients with lower urinary tract symptoms. Neurourol Urodyn 2015. [Epub ahead of print]. [PubMed]
- Nosseir M, Hinkel A, Pannek J. Clinical usefulness of urodynamic assessment for maintenance of bladder function in patients with spinal cord injury. Neurourol Urodyn 2007;26:228-33. [PubMed]
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003;61:37-49. [PubMed]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 2008;9:453-66. [PubMed]
- Weld KJ, Dmochowski RR. Association of level of injury and bladder behavior in patients with post-traumatic spinal cord injury. Urology 2000;55:490-4. [PubMed]
- Panicker JN, Fowler CJ, Kessler TM. Lower urinary tract dysfunction in the neurological patient: clinical assessment and management. Lancet Neurol 2015;14:720-32. [PubMed]
- Lawrenson R, Wyndaele JJ, Vlachonikolis I, et al. Renal failure in patients with neurogenic lower urinary tract dysfunction. Neuroepidemiology 2001;20:138-43. [PubMed]
- National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance. J Spinal Cord Med 2013;36:1-2. [PubMed]
- Winge K. Lower urinary tract dysfunction in patients with parkinsonism and other neurodegenerative disorders. Handb Clin Neurol 2015;130:335-56. [PubMed]
- Kim M, Jung JH, Park J, et al. Impaired detrusor contractility is the pathognomonic urodynamic finding of multiple system atrophy compared to idiopathic Parkinson's disease. Parkinsonism Relat Disord 2015;21:205-10. [PubMed]