Detrusor sphincter dyssynergia: a review of physiology, diagnosis, and treatment strategies
Introduction
The human bladder has two functions—to store and empty urine. Coordination and transition between these functions requires synergy among the detrusor muscle, urinary sphincters, and the central nervous system. When the central neurologic pathways controlling urine storage and emptying are disrupted by injury, inflammation, degenerative process or congenital malformation, the urinary sphincters and detrusor can lose coordination. If the detrusor muscle contracts while the sphincter is activated, bladder outlet obstruction occurs. Detrusor sphincter dyssynergia (DSD) is the urodynamic description of this neurologically induced bladder outlet obstruction. In this manuscript, we will review the physiology, urodynamic diagnostic techniques, and therapeutic treatment options for DSD in the neurogenic bladder patient.
Physiology
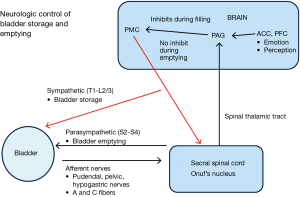
During storage of urine, afferent nerves carry information regarding bladder wall pressure (A fibers) and pain/temperature in the bladder (C fibers) through the pelvic/hypogastric/pudendal nerves to the lumbosacral spinal cord (1). Information is then relayed up the spinal cord spinothalamic tracts to the midbrain periaqueductal grey region. Input from the limbic system and pre-frontal cortex feeds back to the midbrain to either facilitate further bladder storage or to transition to micturition.
When the bladder stores urine, continence is maintained by the internal and external urinary sphincter (EUS) complexes. The internal urinary sphincter is a continuation of the trigone/detrusor muscle which surrounds the bladder neck (2). When the bladder is filling, sympathetic innervation causes the internal sphincter to contract and close the bladder neck. The EUS is a striated muscle group located distal to the internal sphincter. In males, the EUS is a distinct structure, distal to the prostate, and surrounds the membranous urethra. In females, the EUS is located distal to the bladder neck and is comprised of the compressor urethrae muscle, sphincter urethrae muscle, and urethrovaginal sphincter (3). The pudendal nerve fibers control EUS function and are located in Onuf’s nucleus between S2–S4 (4). During urine storage, the pressure in the proximal urethral must be higher than the pressure in the bladder to prevent urinary incontinence. As the bladder fills, hypogastric stimulation of the internal urinary sphincter and pudendal stimulation of the external sphincter progressively raise urethral pressure through a process termed “the guarding reflex” (5). When voiding is to be initiated, inhibition from the midbrain and pre-frontal cortex is lifted and the pontine micturition complex (PMC) inhibits the guarding reflex through the spinobulbospinal tracts. The sympathetic nervous system is inhibited, stimulation of Onuf’s nucleus and the pudendal nerve decreases, and the external sphincter relaxes resulting in lower urethral pressures. Stimulation of the parasympathetic nervous system (S2–S4) then results in micturition (6). Figure 1 summarizes these pathways.
When DSD occurs, the detrusor is contracting against a closed bladder outlet due to involuntary contraction of the urinary sphincter (7). From a physiologic standpoint, this likely represents disruption of spinobulbospinal tract between the PMC and Onuf’s nucleus which results in high urethral closure pressures during a detrusor contraction.
Diagnosing DSD
DSD can only occur in the presence of a neurologic pathology affecting the central nervous system. Contraction of the pelvic floor or urethral sphincter during voiding in a neurologically intact patient should be characterized as “dysfunctional voiding” rather than DSD (7). The diagnosis is made through urodynamic study with or without fluoroscopy via electromyography, voiding cystourethrogram, or urethral profile pressures. It has been the author’s experience to follow up significant findings of DSD on urodynamics with cystoscopy to rule out the possibility of an underlying urethral stricture which can confound urodynamic findings.
EMG
Although needle electrodes placed into the anal sphincter are considered the gold standard for EMG recordings, perineal placement of needles can be challenging and painful for a patient. Perineal surface electrodes are easier to place and more comfortable (8), but the signal may be confounded by lack of adhesion to the skin or by the amount of fat between the muscle and the electrode. Given the potential difficulty in detecting muscle activity by EMG, the ICS Guidelines on urodynamic equipment standardization recommend that EMG during urodynamics should have a high input impedance greater than 100 MOhms, a common-mode rejection (CMRR) greater than 80, and a filtering program to best produce a consistent EMG during testing (9) (Figure 2).
VCUG
There are several reports detailing how voiding cystourethrogram during urodynamics can improve detection of DSD. Yalla et al. described how concomitant urethral narrowing during VCUG correlated with increased sphincter activity on EMG in the setting of a detrusor contraction in DSD patients (10). Blavias et al. also published several reports on using fluoroscopy during urodynamics to assess sphincter function and defined DSD on fluoroscopy as a dilated posterior urethra obstructed by the external sphincter (11). However, a diagnosis of DSD can sometimes be challenging due to technical limitations of the study modalities. To this point, De et al. found that there was only a 60% agreement between EMG and VCUG in 49 patients diagnosed with DSD during their studies (12) (Figure 3).
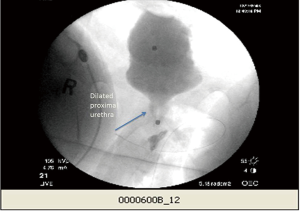
Urethral pressures
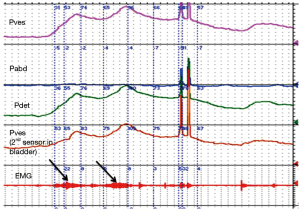
At the University of Michigan, we routinely use urethral pressures as an adjunctive measurement for diagnosing DSD. We use a 7 Fr urodynamic catheter (T-Doc; Wilmington, DE, USA) with independent bladder and urethral pressure sensors with the urethral sensor positioned at the point of maximal pressure in the proximal urethra. We define DSD on urethral pressure measurement as an acute urethral pressure rise >20 cmH2O within 30 seconds prior to or during a voluntary or involuntary detrusor contraction (Figure 4). Using these criteria, in addition to EMG and VCUG definitions of DSD, we examined 67 patients with known DSD previously diagnosed on urodynamics at our institution. We found that 85% of patients had elevated urethral pressures meeting DSD criteria, 75% met EMG criteria, and 55% had VCUG findings consistent with DSD (unpublished data, Lindsey Cox and John Stoffel, University of Michigan). However, these findings contrast to those of Suzuki Bellucci et al. who found urethral pressure measurements had a positive predictive value of 60% and negative predictive value of 56% for the diagnosis of DESD when compared to combining VCUG and EMG (13). At this time, the ICS considers urethral pressures to diagnose DSD to be experimental (14) and more work is needed to determine the role of urethral pressures during urodynamic studies.
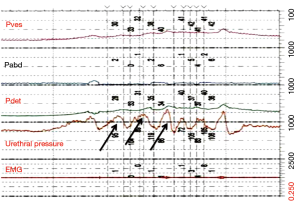
Definitions of DSD
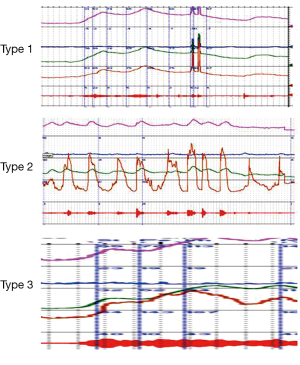
Blaivas et al. proposed criteria for diagnosing DSD during urodynamic testing through characteristic EMG findings (15) (Figure 5).
- Type 1: increasing sphincter activity during detrusor contraction which then ceases and detrusor pressure decreases to allow urination;
- Type 2: clonic contractions intermittently during voiding causing intermittent urinary stream;
- Type 3: continuous sphincter activity during detrusor contraction resulting in inability to void per urethra.
Weld proposed a more simplified characterization based on whether EMG activity was continuous or intermittent (16). Both criteria are inconsistently applied to categorizing DSD today.
Pathophysiology
DSD is commonly found in patients with spinal cord injury and multiple sclerosis but there is no clear relationship to type of DSD and severity of the condition. Work from Schurch et al. suggests that people with an incomplete sensory and motor SCI lesion have more Blaivas type 1 DSD compared to people with complete injuries (17). However, Bellucci et al. did not find a difference in DSD incidence when comparing ambulatory and nonambulatory SCI people (18). Approximately 20–25% of people with multiple sclerosis will develop DSD (19) and although cervical lesions are strongly associated with developing DSD (20), the type of DSD is poorly categorized. It is challenging to develop a phenotype of MS patients with DSD outside of lesion location since other variables such as sex and progressive disease status are not associated with a higher incidence of DSD (21). DSD is also found in up to 50% of infants with spina bifida (22) and other less common conditions affecting the spinobulbospinal tract such as transverse myelitis (23), HTLV-1 (24), and stroke (25).
Diagnosing DSD is important because the condition has potential to cause renal failure through loss of bladder compliance. In 1981, McGuire et al. published findings which clearly associated sustained bladder storage pressures greater than 40 cmH2O in spina bifida patients with increased risk of upper tract damage (26). This concept of the elevated detrusor leak point pressure reflects an increased resistance in the proximal urethra, many times caused by DSD, which does not allow the bladder pressures to decompress through voiding. In spinal cord injury patients, continued voiding with underlying DSD has been associated with the development of hydronephrosis (27). It has been suggested that a continuous DSD phenotype, compared to intermittent DSD, subjects spinal cord injury people to the highest risk of loss of bladder compliance and consequently renal compromise (28).
In the multiple sclerosis population, there is more controversy surrounding associations between DSD and the development of upper tract deterioration. de Sèze and colleagues compiled data from 11 studies consisting of 1,200 MS patients and found a rising incidence of upper tract complications over time for patients symptomatic with MS. These data suggested that upper tract changes were most likely to occur after 6 to 8 years with disease (29). However, two recent studies showed a low incidence of low compliance or upper tract changes in MS patients despite having similar prevalence of DSD (21,30).
DSD has also been thought to be an increased risk for autonomic dysreflexia, particularly in the spinal cord injury population. However, this relationship has been hard to characterize. Liu et al. performed a systematic review of the literature regarding iatrogenic urologic triggers for autonomic dysreflexia and did not find a strong relationship between urodynamic findings of DSD and autonomic dysreflexia. However, the lack of standardization in the description of DSD, urodynamic protocols, and level of injury limited the analysis (31).
Treatment
Clinicians should first identify goals of treatment before initiating any therapy. This author’s recommendation is that practitioners should first determine if the goals of treatment are primarily to improve patient safety, quality of life, or both. Outcomes should then be based on changes in these specific goals and patients should be followed with frequent clinical evaluations to determine efficacy of treatment. Urodynamics should be repeated to determine if interventions directed at improving or resolving DSD remain durable over time.
Pharmacologic agents
Researchers have examined the efficacy of alpha blockers in treated DSD. Chancellor et all gave terazosin 5 mg to 15 normotensive spinal cord injury patients with DSD but could not demonstrate a reduction in voiding pressures on urodynamics (32). In contrast, Stankovich et al. gave tamsulosin 0.4 mg to multiple sclerosis patients with DSD and found the medication significantly improved post void residual measurements and mean volume of voided urine (33). Given that there are little data to support efficacy, alpha blockers are not a recommended therapy for symptomatic DSD.
Similarly, researchers have evaluated anti-spasmodic medications such as baclofen for reducing the severity of DSD in neurogenic bladder patients. Although oral baclofen has notable benefit on treating skeletal muscle spasticity, it has low permeability across the blood brain barrier which limits benefit for treating DSD. Intrathecal delivery of baclofen, in contrast, may directly inhibit interneurons in Onuf’s nucleus which then inhibit the external sphincter (34). In the 1980’s, Leyson et al. reported that intra-thecal baclofen decreased external urethral sphincter resistance and produced a significant reduction of in PVR for 73% of the 125 treated patients within an average of 5 weeks (35). Over the years, there have been additional small trials and case reports supporting baclofen’s efficacy in mitigating DSD (36,37), but there are no randomized trials and long term benefits are not known.
Intravesical instillations of oxybutynin have also been examined as adjunctive treatment for patients with neurogenic overactive bladder and DSD. In theory, reducing uninhibited contractions would decrease the reflexive DSD and thus improve bladder storage and reduce risk of developing low bladder compliance. Like baclofen, there are limited data to support efficacy outside of case series reported in children (38) and limited trials.
Catheterization
Intermittent catheterization (IC) remains a mainstay in managing symptoms/signs related to DSD. When treating progressive hydronephrosis from low bladder compliance or chronic urinary tract infections, this author recommends that the patient empties his or her bladder via catheterization only, and does not attempt to void until the safety issues are stabilized. IC should be performed a sufficient number of times daily to reduce standing volumes contributing to the safety concern and patients should be instructed to pass catheter to level of sphincter and wait for spasms to reduce before continuing to pass the catheter. For patients cathing for DSD induced safety concerns, renal ultrasounds should be performed to gauge effectiveness of the treatment in resolving hydronephrosis and to survey for upper tract stones. If DSD has caused urinary retention resulting in urinary tract symptoms, IC can be performed after voiding to completely empty the bladder. This strategy works particularly well for patients suffering from overflow incontinence, particularly at night. Screening urine cultures in the asymptomatic patient are not recommended for patients performing CIC due to risk of over treating bacterial colonization.
Indwelling catheters can be considered for patients with declining physical condition who are unable to perform IC. For these instances, a suprapubic tube is recommended since indwelling urethral catheters can cause considerable damage in patients with reduced sensation and mobility (39,40). Similar to patients using IC, screening urine cultures in asymptomatic patients with indwelling catheters are not recommended.
Botox
Dykstra et al. first described injecting botulinum A toxin (BTX A) into the external sphincter as treatment for obstructing DSD in spinal cord injury patients. BTX A was injected once a week into the external sphincter in 11 men for a total of three weeks. On follow up, they found that the urethral pressure profile decreased a mean 27 cm of water pressure in seven patients, post void residual decreased a mean 146 cc in eight patients, and that the benefit lasted a mean 50 days (41). Since 1988, there have been several additional small case series reports confirming benefit which can last from 2 to 13 months (42-44). A recent Cochrane meta-analysis of four randomized trials of BTX A treatment for DSD noted that BTX A improves some urodynamic measures such as voiding pressures and PVR after 30 days, although the report notes that the studies had had risk of bias due to inconsistent description of outcome measures (45).
BTX A can be injected into the external sphincter via a cystoscopic or ultrasound guided transperineal approach. The sphincter is usually injected in 2–4 places at the between the 9 to 3 o’clock position across the dorsal aspect of the sphincter. Although the injection technique into the external sphincter is not standardized, most reports suggest using a total dose of 100 units BTX A. If the procedure is performed through a cystoscopy, the injection must be given at least 1 cm deeper than typically performed for bulking agents so that the sphincter muscle is treated and not the submucosa space is injected (46).
Urethral stents
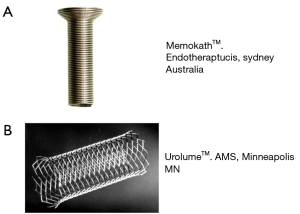
Urethral stents are a controversial treatment option for managing DSD in the neurogenic bladder patient. The goal of the treatment is to place a rigid, non-compressible material across the dysfunction external sphincter to maintain urethral patency. Both temporary and permanent urethral stents have been used to treat DSD. An example of a temporary is the MemoKath™ (Engineers and Doctors, Hornbaek Denmark). This stent is comprised of a temperature sensitive malleable metal alloy which can be expanded in the urethra when heated to 50 degrees C (Figure 6A). The stent contracts when exposed the saline cooled to 5 degrees C, allowing for removal of the device (46). Hamid et al. reported on a 7-year, 25 patients’ experience with the MemoKath and noted that maximum detrusor pressure and post void residual improved 6 months after placement. However, 19 of the 25 patients required stent removal at a mean of 20 months from placement due to stent migration, stone formation, autonomic dysreflexia, and recurrent obstruction (47). Follow up studies at other institutions suggest that the “working life” of a MemoKath may be about 21 months and the long term efficacy may be limited (48).
The Urolume™ (AMS, Minneapolis, MN, USA) is a permanent urethral wall stent made from non-magnetic corrosion resistant stainless steel (Figure 6B). Unlike the MemoKath, it is not temperature sensitive and cannot be molded after it is deployed in the urethra. In 1990, the North American Urolume Multicenter Study Group was formed to investigate stent efficacy in treating DSD. The study enrolled 160 men across 15 centers. Gajewski et al. subsequently reported that the Urolume maintained low PVR, low voiding pressures at 5-year follow up and had low incidence of hydronephrosis or autonomic dysreflexia. Urolume was removed in 13% of patients through an endoscopic resection technique (49). Despite these encouraging results, other centers reported subsequent stent migration (50) and bladder neck obstruction (51) over long term Urolume usage. It has been this author’s experience that long-term Urolume™ usage has time related morbidity and urologic follow up is important to identify subsequent urethral obstruction, stone formation, and urethral erosion.
Sphincterotomy
Outside of urinary diversion, endoscopic urethral sphincterotomy is the most invasive treatment for symptomatic DSD. Similar to BTX A, the goal of the treatment is to reduce outlet obstruction by impairing external sphincter function and to create low bladder storage pressures. The external sphincter can be resected either with electrocautery or via a cold knife and treatment is focused at the 12 o’clock location to limit hemorrhage. Nonetheless, the procedure can cause significant bleeding and a large catheter >20 Fr, is usually required for several days to apply transurethral pressure at the resection site. Additional complications include urinary extravasation, so VCUG is usually recommended during the post procedural urodynamic follow up. The practitioner should also be aware that bladder neck obstruction may become more prominent after external sphincterotomy which may need to be subsequently treated. The procedure is performed mostly in male DSD patients, since a successful treatment usually requires patients to wear an external urinary collection device such as a condom catheter. Pan et al. reported on outcomes for 84 patients. Mean duration of successful treatment was 81 months. Although 68% of patients experienced subsequent recurrent UTI, symptomatic DSD, or upper tract dilation on imaging, no patient experienced progressive renal failure (52). Vainrib et al. noted similar outcomes in their review of 97 patients treated over a 40-year follow-up. In this study, patients required a mean three repeat procedures and experienced a greater than 50% long term success rate (53).
Conclusions
DSD is a condition is that diagnosed via urodynamics in neurogenic bladder patients. There is no uniform classification system for DSD, but it is generally thought of as either intermittent or continuous depending upon the duration of sphincteric activity during detrusor contraction. Patients with DSD may be at risk for autonomic dysreflexia, recurrent urinary tract infections, or upper tract compromise if not clinically followed. DSD has been successfully treated with botulinum toxin A injections and transurethral sphincterotomy, although more research is needed to best identify optimal candidates and to reduce procedural morbidity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Yoshimura N, Kaiho Y, Miyazato M, et al. Therapeutic receptor targets for lower urinary tract dysfunction. Naunyn Schmiedebergs Arch Pharmacol 2008;377:437-48. [PubMed]
- Ashton-Miller JA, DeLancey JO. Functional anatomy of the female pelvic floor. Ann N Y Acad Sci 2007;1101:266-96. [PubMed]
- Jung J, Ahn HK, Huh Y. Clinical and functional anatomy of the urethral sphincter. Int Neurourol 2012;16:102-6.
- Pullen AH, Tucker D, Martin JE. Morphological and morphometric characterisation of Onuf's nucleus in the spinal cord in man. J Anat 1997;191:201-13. [PubMed]
- Fowler CJ. Integrated control of lower urinary tract-clinical perspective. Br J Pharmacol 2006;147:S14-24. [PubMed]
- Castro-Diaz D, Taracena Lafuente JM. Detrusor-sphincter dyssyn- ergia. Int J Clin Pract Suppl 2006;151:17-21. [PubMed]
- Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Subcommittee of the International Continence Society. Neurourol Urodyn 2002;21:167-78. [PubMed]
- Spettel S, Kalorin C, De E. Combined diagnostic modalities improve detection of detrusor external sphincter dyssynergia. ISRN Obstet Gynecol 2011;2011:323421.
- Gammie A, Bosch R, Djurhuus JC, et al. Do we need better methods of assessing urethral function: ICI-RS 2013? Neurourol Urodyn 2014;33:587-90. [PubMed]
- Yalla SV, Blunt KJ, Fam BA, et al. Detrusorurethral sphincter dyssynergia. J Urol 1977;118:1026-9. [PubMed]
- Blaivas JG, Chaikin DC, Chancellor MB, et al. Detrusor-external sphincter dyssynergia. J Urol 1981;125:542-4. [PubMed]
- De EJ, Patel CY, Tharian B, et al. Diagnostic discordance of electromyography (EMG) versus voiding cystourethrogram (VCUG) for detrusor-external sphincter dyssynergy (DESD). Neurourol Urodyn 2005;24:616-21. [PubMed]
- Suzuki Bellucci CH, Wöllner J, Gregorini F, et al. External urethral sphincter pressure measurement: an accurate method for the diagnosis of detrusor external sphincter dyssynergia? PloS One 2012;7:e37996. [PubMed]
- Lose G, Griffiths D, Hosker G, et al. Standardization of urethral pressure measurement: report from the Standardization Sub-committee of the International Continence Society. Neurourol Urodyn 2002;21:258-60. [PubMed]
- Blaivas JG, Sinha HP, Zayed AA, et al. Detrusor-external sphincter dyssynergia: a detailed electromyographic study. J Urol 1981;125:545-8. [PubMed]
- Weld KJ, Wall BM, Mangold TA, et al. Influences on renal function in chronic spinal cord injured patients. J Urol 2000;164:1490-3. [PubMed]
- Schurch B, Schmid DM, Karsenty G, et al. Can neurologic examination predict type of detrusor sphincter-dyssynergia in patients with spinal cord injury? Urology 2005;65:243-6. [PubMed]
- Bellucci CH, Wöllner J, Gregorini F, et al. Acute spinal cord injury--do ambulatory patients need urodynamic investigations? J Urol 2013;189:1369-73. [PubMed]
- Stoffel JT. Contemporary management of the neurogenic bladder for multiple sclerosis patients. Urol Clin North Am 2010;37:547-57. [PubMed]
- Araki I, Matsui M, Ozawa K, et al. Relationship of bladder dysfunction to lesion site in multiple sclerosis. J Urol 2003;169:1384-7. [PubMed]
- Cox L, Cameron AP, Wittman D, et al. Analysis of Urinary Symptoms and Urodynamic Findings in Multiple Sclerosis Patients by Gender and Disease Subtype. J Neurol 2015;1:1-5.
- Bauer SB, Dieppa RA, Labib KK, et al. Predictive value of urodynamic evaluation in newborns with myelodysplasia. JAMA 1984;252:650-2. [PubMed]
- Gliga LA, Lavelle RS, Christie AL, et al. Urodynamics findings in transverse myelitis patients with lower urinary tract symptoms: Results from a tertiary referral urodynamic center. Neurourol Urodyn 2015;34:507-12. [PubMed]
- Castro NM, Freitas DM, Rodrigues W, et al. Urodynamic features of the voiding dysfunction in HTLV-1 infected individuals. Int Braz J Urol 2007;33:238-44; discussion 244-5. [PubMed]
- Meng NH, Lo SF, Chou LW, et al. Incomplete bladder emptying in patients with stroke: is detrusor external sphincter dyssynergia a potential cause? Arch Phys Med Rehabil 2010;91:1105-9. [PubMed]
- McGuire EJ, Woodside JR, Borden TA, et al. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol 1981;126:205-9. [PubMed]
- Killorin W, Gray M, Bennett JK, et al. The value of urodynamics and bladder management in predicting upper urinary tract complications in male spinal cord injury patients. Paraplegia 1992;30:437-41. [PubMed]
- Bacsu CD, Chan L, Tse V. Diagnosing detrusor sphincter dyssynergia in the neurological patient. BJU Int 2012;109 Suppl 3:31-4. [PubMed]
- de Sèze M, Ruffion A, Denys P, et al. The neurogenic bladder in multiple sclerosis: review of the literature and proposal of management guidelines. Mult Scler 2007;13:915-28. [PubMed]
- Fletcher SG, Dillon BE, Gilchrist AS, et al. Renal deterioration in multiple sclerosis patients with neurovesical dysfunction. Mult Scler 2013;19:1169-74. [PubMed]
- Liu N, Zhou M, Biering-Sørensen F, et al. Iatrogenic urological triggers of autonomic dysreflexia: a systematic review. Spinal Cord 2015;53:500-9. [PubMed]
- Chancellor MB, Erhard MJ, Rivas DA. Clinical effect of alpha-1 antagonism by terazosin on external and internal urinary sphincter function. J Am Paraplegia Soc 1993;16:207-14. [PubMed]
- Stankovich EIu, Borisov VV, Demina TL. Tamsulosin in the treatment of detrusor-sphincter dyssynergia of the urinary bladder in patients with multiple sclerosis. Urologiia 2004;4:48-51. [PubMed]
- Blok BF, Holstege G. Ultrastructural evidence for a direct pathway from the pontine micturition center to the parasympathetic preganglionic motoneurons of the bladder of the cat. Neurosci Lett 1997;222:195-8. [PubMed]
- Leyson JF, Martin BF, Sporer A. Baclofen in the treatment of detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1980;124:82-4. [PubMed]
- Meythaler JM, Steers WD, Tuel SM, et al. Continuous intrathecal baclofen in spinal cord spasticity. A prospective study. Am J Phys Med Rehabil 1992;71:321-7. [PubMed]
- Bushman W, Steers WD, Meythaler JM. Voiding dysfunction in patients with spastic paraplegia: urodynamic evaluation and response to continuous intrathecal baclofen. Neurourol Urodyn 1993;12:163-70. [PubMed]
- Humblet M, Verpoorten C, Christiaens MH, et al. Long-term outcome of intravesical oxybutynin in children with detrusor-sphincter dyssynergia: with special reference to age-dependent parameters. Neurourol Urodyn 2015;34:336-42. [PubMed]
- Stoffel JT, McGuire EJ. Outcome of urethral closure in patients with neurologic impairment and complete urethral destruction. Neurourol Urodyn 2006;25:19-22. [PubMed]
- Feifer A, Corcos J. Contemporary role of suprapubic cystostomy in treatment of neuropathic bladder dysfunction in spinal cord injured patients. Neurourol Urodyn 2008;27:475-9. [PubMed]
- Dykstra DD, Sidi AA, Scott AB, et al. Effects of botulinum A toxin on detrusor-sphincter dyssynergia in spinal cord injury patients. J Urol 1988;139:919-22. [PubMed]
- Schurch B, de Seze M, Denys P, et al. Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: Results of a single treatment, randomized, placebo controlled 6-month study. J Urol 2005;174:196-200. [PubMed]
- Gallien P, Robineau S, Verin M, et al. Treatment of detrusor sphincter dyssynergia by transperineal injection of botulinum toxin. Arch Phys Med Rehabil 1998;79:715-7. [PubMed]
- Kuo HC. Therapeutic outcome and quality of life between urethral and detrusor botulinum toxin treatment for patients with spinal cord lesions and detrusor sphincter dyssynergia. Int J Clin Pract 2013;67:1044-9. [PubMed]
- Utomo E, Groen J, Blok BF. Surgical management of functional bladder outlet obstruction in adults with neurogenic bladder dysfunction. Cochrane Database Syst Rev 2014;5:CD004927. [PubMed]
- Mahfouz W, Corcos J. Management of detrusor external sphincter dyssynergia in neurogenic bladder. Eur J Phys Rehabil Med 2011;47:639-50. [PubMed]
- Hamid R, Arya M, Wood S, et al. The use of the Memokath stent in the treatment of detrusor sphincter dyssynergia in spinal cord injury patients: a single-centre seven-year experience. Eur Urol 2003;43:539-43. [PubMed]
- Mehta SS, Tophill PR. Memokath stents for the treatment of detrusor sphincter dyssynergia (DSD) in men with spinal cord injury: The Princess Royal Spinal Injuries Unit 10-year experience. Spinal Cord 2006;44:1-6. [PubMed]
- Gajewski JB, Chancellor MB, Ackman CF, et al. Removal of UroLume endoprosthesis: experience of the North American Study Group for detrusor-sphincter dyssynergia application. J Urol 2000;163:773-6. [PubMed]
- Wilson TS, Lemack GE, Dmochowski RR. UroLume stents: lessons learned. J Urol 2002;167:2477-80. [PubMed]
- Abdul-Rahman A, Ismail S, Hamid R, et al. A 20-year follow-up of the mesh wallstent in the treatment of detrusor external sphincter dyssynergia in patients with spinal cord injury. BJU Int 2010;106:1510-3. [PubMed]
- Pan D, Troy A, Rogerson A, et al. Long-term outcomes of external sphincterotomy in a spinal injured population. J Urol 2009;181:705-9. [PubMed]
- Vainrib M, Reyblat P, Ginsberg DA. Long-term efficacy of repeat incisions of bladder neck/external sphincter in patients with spinal cord injury. Urology 2014;84:940-5. [PubMed]