
Significance of hyperhomocysteinaemia as an effective marker for vasculogenic erectile dysfunction: a cross-sectional study
Introduction
Erectile dysfunction (ED) refers to a recurrent or persistent failure to achieve and maintain an effective erection to perform satisfactory sexual activity for at least 6 months (1). It is one of the most common male-related diseases that negatively impacts on the quality of life for patients and their partners (2,3). Epidemiologic studies have reported a high incidence and prevalence of ED all over the word, afflicting more than 150 million population and is postulated to affect 322 million people by 2025 (4). Penile erection involves a series of complicated and coordinated physiologic activities, including nerve electrophysiology and hemodynamic changes (5). Consequently, due to different etiologies, including vascular, neurogenic, hormonal, psychogenic, cavernosa, and anatomical abnormalities, the pathophysiology of ED is multifactorial (6). Since corpus cavernosum is believed to have a special vascular webbing, vascular reasons dominate the etiology of vasculogenic ED. Furthermore, endothelial dysfunction is one of the early changes of vascular pathologies such as angiocardiopathy (7). Therefore, ED, especially vasculogenic ED, should serve as an early clinical feature of cardiovascular diseases (CVD).
Experimental models have elucidated that an emerging risk factor for endothelial dysfunction is an elevation of the amino acid, homocysteine [i.e., hyperhomocysteinaemia (HHcy)] (8). Homocysteinemia (Hcy), as a strong and independent predictor for atherosclerosis progression and impaired cavernosal perfusion, is a sulfur-containing amino acid that is generated from metabolic demethylation of dietary methionine (9). There are two metabolic pathways for Hcy (10,11). In the remethylation pathway, Hcy is converted back to methionine by 5,10-methylenetetrahydrofolate reductase or betaine homocysteine methyltransferase, which needs folate or vitamin B12 as a cofactor. This pathway occurs when there are low methionine levels. In the trans-sulfuration pathway, when there is excess methionine after meals, it is alternatively transformed to cystathionine by cystathionine-β-synthase (CBS), whose cofactor is vitamin B6 (12). Previous literatures have approved that HHcy is strongly correlated with vascular endothelial damage, which might affect vascular physiologic functions (13). Consequently, elevated Hcy was found to be able to inhibit NO-synthase, which is responsible of nitric oxide (NO) production, a vasodilatation mediator, whose deficiency plays an important role in the pathogenesis of atherosclerosis (14).
Up to date, lots of studies have evaluated the relationship between HHcy levels and vascular system disorders, however, relatively few studies evaluating the association between serum Hcy levels and vasculogenic ED pathogenesis. Although the association between HHcy and ED has been demonstrated, we believe that the association between HHcy and vasculogenic ED cannot be generalized to all patients with ED. The potential relationship between vascular ED and CVD has led us to speculate that vasculogenic ED may be linked to HHcy as an indicator of chronic vascular risk. Therefore, we combined the Rigiscan with Doppler ultrasound to evaluate patients with vasculogenic ED and aimed to investigated the correlation between Hcy and vasculogenic ED in the present study. Above all, this study will help us to define the diagnostic and prognostic value of homocysteine in vasculogenic ED.
We present the following article in accordance with the STARD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-21-953/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for this study was obtained from the Institutional Ethics Committee of the Center for Reproductive Medicine of Shandong University (No.2021-085), and all participants provided the written informed consent before participating in the study.
From January 2020 to Jun 2021, patients complaining of ED were admitted to the Urology Department at the Center for Reproductive Medicine of Shandong University and enrolled in the study. All participants were asked to complete the IIEF-5 questionnaire, and ED was defined as a score ≤21 of the IIEF questionnaire (15). After completing the questionnaire, only 119 people from a total of 244 patients scored less than 21.
Participants with the following conditions were excluded from the study; cardiovascular and/or cerebrovascular diseases, metabolic syndromes, tumors, inflammatory conditions, hematologic diseases, psychiatric disorders, hypogonadism, neurological disorders, penile fibrosis, autoimmune diseases, failure to obtain their hemograms, or those using medications that may affect erectile function.
Study design
Participants were subjected to a detailed history (anamnesis), physical examination, laboratory evaluations, erectile function examination [i.e., nocturnal penile tumescence (NPT)] and penile colour Doppler ultrasonography (pDUS). Blood analysis, which included the lipid panel [total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides (TG)], fasting blood glucose (FBG), Hcy, testosterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (E2), prolactine (PRL) levels were determined using a biochemical analyzer at the laboratory of the Center for Reproductive Medicine of Shandong University (Modular Analytics, Roche, Mannheim, Germany). Venous blood samples from all participants were collected from the vein in the morning after overnight fasting and processed within 1 h in order to prevent temperature and time dependent increase in plasma Hcy. In clinical studies, Hcy levels were classified into normal (Hcy ≤15 µmol/L) and HHcy (c) with the cut-off value of 15 µmol/L (16).
Erectile function was monitored in all participants using the Rigiscan® device (Timm Medical Technologies, Inc. Eden Prairie, MN55344, USA). All subjects were forbidden from napping, drinking caffeine or alcohol, strenuous exercise and evacuated the bladder prior to going to sleep in order to ensure a restful night sleep. During the three consecutive nights of NPT tests, they were advised to avoid “casus fortuitous” to ensure result accuracy. Normal nocturnal erection was defined as at least three tumescence periods lasting more than 10 minutes with 60% tip rigidity (17), or penile circumference extended to more than 3 cm at the base or 2 cm at the tip (18).
Furthermore, pDUS (Sonicaid 9900, Medison, Seoul, Korea) was performed on patients who had ED complaints by the same CDFI-certified urologist following standard protocols using a 6–12 MHz high-frequency ultrasound probe. Cavernous arteries of patients were measured at baseline conditions after tactile stimulation. Subsequently, phentolamine 0.1 mL (0.5 mg L-1; Novartis, Beijing, China) and papaverine 0.7 mL (30 mg L-1; Henrui Medicine, Lianyungang, China) were injected into the penis base. Optimal vascular flow parameters, including peak-systolic velocity (PSV) and end-diastolic velocity (EDV) were registered at baseline within 20 min post-injection. Participants were left alone in the isolated and quiet room during the evaluations to avoid the loss of concentration. The diagnostic criteria for vasculogenic ED included arterial insufficiency (PSV <25 cm/s and EDV <5 cm/s), veno-occlusive dysfunction [EDV ≥5 cm/s, and PSV ≥25 cm/s, resistance index (RI) <0.6], and mixed vascular disorder (PSV <25 cm/s, EDV ≥5 cm/s, RI <0.6) (19,20).
Statistical analysis
Analyses were performed using Empower (R) (www.empowerstats.com, X&Y solutions, Inc Boston, MA) and R (http://www.R-project.org). The power analyses were conducted with a two-sided test in two-sample T-test under the significance level of 0.05, using PASS 11.0 (NCSS, LLC. Kaysville, Utah). Normality was assessed by Kolmogorov-Smirnov test. Continuous variables were expressed as the mean ± standard deviation (SD) or median [interquartile range (IQR)], and frequency (percentage) was used to describe the count data. Data and laboratory results were compared using Student’s t test or Mann-Whitney U test, and the chi-square test or Fisher’s exact chi-square was used for categorical variables Univariate analyses were performed to determine the significance of variables. Although these factors, including FBG, HDL, LDL, TG and TC, have no significant effect on vasogenic ED events, they are potential risk factors for CVD. Therefore, we used multivariate regression analyses to estimate the independent relationship between Hcy concentrations and vasculogenic ED after adjusting for these potential confounders. Then, we performed regression analysis to fit the smoothing curve. Receiver operating characteristic (ROC) analysis was used to estimate the diagnostic value of Hcy for vasculogenic ED.
Results
Based on IIEF-5 questionnaire, history, NPT, and pDUS, a total of 119 patients with ED were identified (Figure 1). Demographics and basic statistical descriptions of all 119 patients are summarized in Table 1. Patients with vasculogenic ED were older on average compared to those with non-vasculogenic ED (41.02±7.42 versus 33.18±7.22, P<0.01). Serum Hcy concentrations were generally elevated in patients with vasculogenic ED than in those with non-vasculogenic ED. Mean (± SD) Hcy levels for vasculogenic ED and non-vasculogenic ED were 22.91±5.85 and 16.31±5.23 µmol/L (P<0.01), respectively. In addition, the proportion of hypertension (P<0.01) was also identified to have a statistically significant difference in the two groups. Except for age, Hcy and hypertension, there were no significant differences in other risk factors related to ED between the two groups.
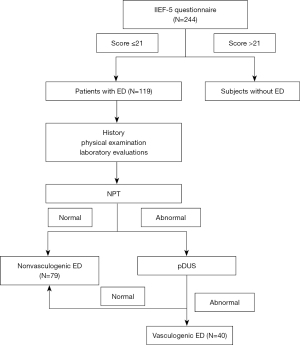
Table 1
| Characteristics | Non-vasculogenic ED (N=79) | Vasculogenic ED (N=40) | P value |
|---|---|---|---|
| Age (years) | 33.18±7.22 | 41.02±7.42 | <0.01 |
| FBG (mmol/L) | 4.66±0.98 | 5.09±1.66 | 0.08 |
| TC (mmol/L) | 3.92±0.81 | 4.07±0.94 | 0.37 |
| HDL (mmol/L) | 1.14±0.28 | 1.13±0.21 | 0.78 |
| LDL (mmol/L) | 2.50±0.75 | 2.61±0.94 | 0.53 |
| LH (mIU/mL) | 4.75±2.48 | 4.39±2.10 | 0.43 |
| E2 (pg/mL) | 56.59±24.85 | 54.91±23.99 | 0.73 |
| Hcy (μmol/L) | 16.31±5.23 | 22.91±5.85 | <0.01 |
| Testosterone (ng/mL) | 4.31±1.30 | 3.97±1.05 | 0.16 |
| PRL (ng/mL), median (IQR) | 12.99 (9.67–16.23) | 15.13 (11.61–21.35) | 0.09 |
| TG (mmol/L), median (IQR) | 0.95 (0.64–1.54) | 0.99 (0.56–1.39) | 0.54 |
| FSH (mIU/mL), median (IQR) | 5.00 (3.49–6.80) | 4.45 (2.68–5.81) | 0.07 |
| Progesterone (ng/mL), median (IQR) | 0.87 (0.61–1.17) | 0.85 (0.57–1.09) | 0.58 |
| HHcy | <0.01 | ||
| No | 48 (60.76%) | 5 (12.50%) | |
| Yes | 31 (39.24%) | 35 (87.50%) | |
| Trauma | 0.51 | ||
| No | 67 (84.81%) | 32 (80.00%) | |
| Yes | 12 (15.19%) | 8 (20.00%) | |
| Hypertension (%) | <0.01 | ||
| No | 69 (87.34%) | 24 (60%) | |
| Yes | 10 (12.66%) | 16 (40%) | |
| Smoking (%) | 0.64 | ||
| No | 54 (68.35%) | 29 (72.50%) | |
| Yes | 25 (31.65%) | 11 (27.50%) |
ED, erectile dysfunction; E2, estradiol; FBG, fasting blood glucose; FSH, follicle-stimulating hormone; Hcy, homocysteine; HHcy, hyperhomocysteinaemia; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LH, luteinizing hormone; PRL, prolactine; IQR, interquartile range; TG, triglycerides; TC, total cholesterol.
Among the ED patients, univariate regression analysis was carried out to assess whether risk factors were associated with vasculogenic ED events (Table S1). Univariate models identified age and Hcy to be significantly associated with vasculogenic ED events [odds ratio (OR): 1.14, 95% confidence interval (CI): 1.08 to 1.21 and OR: 1.22, 95% CI: 1.12 to 1.31]. Meanwhile, using normal Hcy group for reference, HHcy group was positively associated with risk of vasculogenic ED event (OR: 10.84, 95% CI: 3.83 to 30.67, P<0.01). The risk of vasculogenic ED occurred to hypertension increased to 4.6 times as compared with non-hypertension (OR: 4.60, 95% CI: 1.84 to 11.50, P<0.01). Then, multivariate regression models adjusted for possible confounding factors (Table 2): model 1 adjusted for age and hypertension, while model 2 adjusted for age, FBG, TC, HDL, LDL, TG and hypertension. The multivariate regression analysis found that Hcy was independently and significantly correlated with the occurrence of vasculogenic ED. It is considered to be HHcy when the level of Hcy exceeded 15 µmol/L. Patients with HHcy had 13.42 times the odds of vasculogenic ED compared with patients without HHcy (adjusted OR in model 2 =13.42, 95% CI: 3.78 to 47.64, P<0.01). Interestingly, in ED patients with HHcy, the positive correlation between HHcy levels and the occurrence of vasculogenic ED was further confirmed in Table 2 (adjusted OR in model 2 =1.24, 95% CI: 1.04 to 1.48, P=0.01).
Table 2
| Risk factor(s) | Mean + SD/N (%) | Unadjust | Model I | Model II | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | ||||
| Hcy (μmol/L) | 18.53±6.26 | 1.22 | 1.12, 1.31 | <0.01 | 1.25 | 1.14, 1.38 | <0.01 | 1.25 | 1.13, 1.38 | <0.01 | ||
| HHcy | ||||||||||||
| No | 53 (44.54%) | Ref | Ref | Ref | ||||||||
| Yes | 66 (55.46%) | 10.84 | 3.83, 30.67 | <0.01 | 11.39 | 3.53, 36.74 | <0.01 | 13.42 | 3.78, 47.64 | <0.01 | ||
| HHcy levels | ||||||||||||
| Hcy ≤15 (μmol/L) | 12.75±1.49 | 1.72 | 0.74, 4.04 | 0.21 | 1.79 | 0.62, 5.22 | 0.28 | 1.98 | 0.56, 7.06 | 0.29 | ||
| Hcy >15 (μmol/L) | 23.17±4.52 | 1.13 | 1.05, 1.42 | 0.01 | 1.23 | 1.05, 1.44 | 0.01 | 1.24 | 1.04, 1.48 | 0.01 | ||
Model I: Adjusted for age and hypertension; Model II: Adjusted for age, FBG, TC, HDL, LDL, TG, and hypertension. CI, confidence interval; ED, erectile dysfunction; Hcy, homocysteine; HHcy, hyperhomocysteinaemia; OR, odds ratio Ref, reference; SD, standard deviation.
Penile Doppler ultrasonography findings of the two ED groups are presented in Table 3. PSV scores for the two groups were 33.19±15.91 and 17.15±7.42 cm/s (P<0.01) while EDV scores were 0.24±3.82 and 2.92±3.07 cm/s (P<0.01). The smoothing curve showed that, after adjusting for confounders, including age, FBG, TC, HDL, LDL, TG and hypertension, there is a negative linear correlation between PSV, EDV and Hcy (Figure 2). After adjusting for the same confounders, multivariable analyses elucidated a strong association between Hcy levels and PSV in Table 4 (β: −0.48, 95% CI: −0.91 to −0.05, P=0.04). However, a significant inverse correlation was not detected between EDV and Hcy levels in vasculogenic ED (β: −0.13, 95% CI: −0.30 to 0.03, P=0.13).
Table 3
| Characteristics | Non-vasculogenic ED (N=79) | Vasculogenic ED (N=40) | P value |
|---|---|---|---|
| PSVb (cm/s) | 33.19±15.91 | 17.15±7.42 | <0.01 |
| EDVb (cm/s) | 0.24±3.82 | 2.92±3.07 | <0.01 |
bValue: pertain to statistically significant difference by Mann-Whitney U test. ED, Erectile dysfunction; PSV, peak-systolic velocity; EDV, end-diastolic velocity.
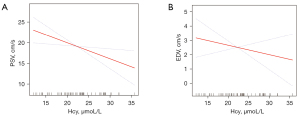
Table 4
| PSV (cm/s) | EDV (cm/s) | ||||||
|---|---|---|---|---|---|---|---|
| β | (95% CI) | P | β | (95% CI) | P | ||
| Unadjust | −0.41 | −0.79, −0.03 | 0.04 | 0.13 | −0.29, 0.03 | 0.12 | |
| Model I | −0.40 | −0.80, −0.00 | 0.05 | −0.10 | −0.26, 0.06 | 0.21 | |
| Model II | −0.48 | −0.91, −0.05 | 0.04 | −0.13 | −0.30, 0.03 | 0.13 | |
Model I: Adjusted for age and hypertension; Model II: Adjusted for age, FBG, TC, HDL, LDL, TG and hypertension. ED, erectile dysfunction; EDV, end-diastolic velocity; Hcy, homocysteine; PSV, peak-systolic velocity.
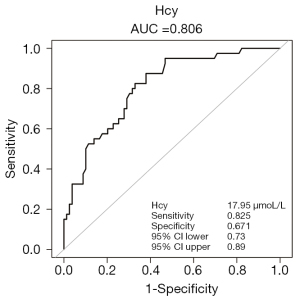
To elucidate the possibility of the Hcy as a diagnostic and prognostic marker, the receiver operating characteristic (ROC) curve of Hcy for predicting vasculogenic ED were calculated in ED patients. In the ROC regression analysis, the outcome verified Hcy ≥17.95 µmol/L as a cut-off value for potential vasculogenic ED [area under the curve (AUC): 0.81, 95% CI: 0.73–0.89]. The best threshold had a sensitivity of 82.50% and specificity of 67.09% (Figure 3).
Discussion
Erectile dysfunction, one of the most prevalent sexual disorders, is considered to interfere with the quality of life for millions of couples around the world (21). Incidences of ED increases with age and is associated with multiple comorbidities. In addition, vascular, hormonal, lifestyle, neurologic, and psychological factors all play important roles in the etiology of ED (22).
Penile erection is currently recognized as an event that is modulated by nerves, vessels and hormones (23). Vasculogenic ED, as a precursor of cardiovascular disease (CVD), is an independent biomarker of CVD risk and may reveal the presence of subclinical vascular diseases in men (24). Endothelial dysfunction plays an integral role in cardiovascular and peripheral vascular diseases. The vascular endothelium, a monolayer of cells distributed on the surface of vascular lumens, can regulate local vascular functions and affect vascular tone, inflammation, and permeability in the form of paracrine and endocrine (25). Therefore, we infer that the impaired cavernous endothelial cells may be among the most important pathological mechanisms in vasculogenic ED.
To the best of our knowledge, HHcy has been known as an independent risk factor for vascular diseases (26). A significant association between elevated plasma Hcy levels and atherosclerotic vascular disease was confirmed by a prospective meta-analysis of study (27). HHcy has been shown to lead to endothelial dysfunction and injury in vivo and in vitro. Potential mechanisms of HHcy induced endothelial dysfunction may be due to increased oxidative stress, decreased bioavailability of endodermal nitric oxide (NO), promoting endothelial inflammation and apoptotic cell death (28). Based on a previously established relationship between HHcy and cardiovascular events, Hcys has been introduced as one of the mechanisms involved in the pathogenesis of ED. In an experimental model, HHcy has been identified as a novel risk factor for ED (29). In the last few decades, the relationships between ED and HHcy have been determined. However, studies focused on ED without distinguishing between vasculogenic ED and non-vasculogenic ED.
Several previous studies have confirmed significantly elevated homocysteine levels in “ED” patients than in “controls” (26,30). Elevated Hcy levels are, therefore, potential emerging risk factors for ED pathogenesis. However, as we all know, most literatures have reported the relationship between Hcy and ED, but few studies have reported on the relationship between Hcy and vasculogenic ED. In this study, the improvement of our study aimed to further investigate the association between Hcy and vasculogenic ED. Univariate analyses confirmed that, with increases in age and Hcy levels, incidences of vasculogenic ED were higher than those of non-vasculogenic ED. Multivariate analysis showed that HHcy levels were independently associated with the occurrence of vasculogenic ED events (Table 2). This outcome coincides with the fact that vasculogenic ED is an early manifestation of endothelial dysfunction (31). Importantly, we also observed that ED patients with HHcy were significantly more likely to be diagnosed with vasculogenic ED comparing with patients without HHcy.
pDUS, which uses high resolution real-time gray scale ultrasonography and color Doppler flow imaging technology, is a crucial method for the diagnosis and differential diagnosis of vasculogenic ED. PSV and EDV are important indicators of penile hemodynamics and are used in evaluating the vascular status of ED patients. Interestingly, after adjusting for potential confounding factors, multivariate regression analysis and smoothing curve revealed that PSV was negatively correlated with Hcy in vasculogenic ED. However, there was no significant correlation between Hcy and EDV. The dose-dependent relationship between Hcy levels and vasculogenic ED has also been revealed by penile Doppler ultrasound studies. In addition, the ROC curve of Hcy for predicting vasculogenic ED demonstrated that the cut-off value of Hcy was 17.95 µmol/L with a sensitivity of 82.50% and a specificity of 67.09%. These results suggest that those ED patients with Hcy values 17.95 µmol/L have an increased risk of vasculogenic ED, and the diagnostic sensitivity and specificity of HHcy in diagnosing vascular ED were acceptable. In summary, HHcy is a potential indicator to predict and diagnose vasculogenic ED. Most important of all, HHcy, an independent risk factor for vascular disease, can be easily measured by blood biochemistry at a relatively low cost. In most patients, they are reluctant to undergo pDUS, because of the examination will bring trauma to the private parts of patients, and Hcy testing can help reduce trauma caused by intracavernous drug injections.
We should also be concerned about some limitations in this study. First, the single-center nature of the study may have an impact on external validation. Second, the relatively small sample size may restrict result generalizability. Third, we did not perform cavernosography and repeated hemodynamic assessments to ensure diagnostic accuracy. In addition, a series of comprehensive blinded validation studies are warranted to confirm the clinical effects of Hcy.
In conclusion, our results confirm the positive correlation between HHcy and vasculogenic ED, Hcy was an independent risk factor for vasculogenic ED. In addition, penile hemodynamics assessed by penile Doppler ultrasonography, also reconfirmed that elevated plasma Hcy was inversely associated with PSV in vasculogenic ED patients. Thus, Hcy could potentially be monitored as a useful marker to predict vasculogenic ED. However, these conclusions came from a limited cohort of ED patients, and our results should be confirmed by additional studies. To the best of our knowledge, additional large-scale and real-world studies are required in order to generalize the conclusion to the whole crowd.
Acknowledgments
All authors would like to thank the editorial team of Home for Researchers (www.home-for-researchers.com) for the language assistance.
Funding: This work was supported by grants from Rongxiang Regenerative Medicine Foundation of Shandong University (No. 2019SDRX-xx).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-21-953/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-21-953/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-21-953/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval for this study was obtained from the Institutional Ethics Committee of the Center for Reproductive Medicine of Shandong University (No.2021-085), and all participants provided the written informed consent before participating in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Guo LQ, Liu YQ, Sun WD, et al. Significance of platelet distribution width as a severity marker of erectile dysfunction. Andrologia 2017;49. [Crossref] [PubMed]
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and Its Medical and Psychosocial Correlates: Results of the Massachusetts Male Aging Study. J Urol 1994;151:54-61. [Crossref] [PubMed]
- Burchardt M, Burchardt T, Baer L, et al. Hypertension Is Associated with Severe Erectile Dysfunction. J Urol 2000;164:1188-91. [Crossref] [PubMed]
- de Boer BJ, Bots ML. Erectile dysfunction in primary care: prevalence and patient characteristics. The ENIGMA study. Int J Impot Res 2004;16:358-64. [Crossref] [PubMed]
- Sié P, Meguira B, Bouissou F, et al. Plasma levels of heparin cofactor II in nephrotic syndrome of children. Nephron 1988;48:175-6. [Crossref] [PubMed]
- Chew KK, Bremner A, Stuckey B, et al. Is the relationship between cigarette smoking and male erectile dysfunction independent of cardiovascular disease? Findings from a population-based cross-sectional study. J Sex Med 2009;6:222-31. [Crossref] [PubMed]
- Aizawa K, Shoemaker JK, Overend TJ, et al. Metabolic syndrome, endothelial function and lifestyle modification. Diab Vasc Dis Res 2009;6:181-9. [Crossref] [PubMed]
- Ungvari Z, Csiszar A, Bagi Z, et al. Impaired Nitric Oxide-Mediated Flow-Induced Coronary Dilation in Hyperhomocysteinemia. The American Journal of Pathology 2002;161:145-53. [Crossref] [PubMed]
- Rasouli ML, Nasir K, Blumenthal RS, et al. Plasma homocysteine predicts progression of atherosclerosis. Atherosclerosis 2005;181:159-65. [Crossref] [PubMed]
- Mayengbam S, Raposo S, Aliani M, et al. Oral exposure to the anti-pyridoxine compound 1-amino D-proline further perturbs homocysteine metabolism through the transsulfuration pathway in moderately vitamin B(6) deficient rats. J Nutr Biochem 2015;26:241-9. [Crossref] [PubMed]
- Selhub J. Homocysteine metabolism. Annu Rev Nutr 1999;19:217-46. [Crossref] [PubMed]
- Finkelstein JD. Pathways and regulation of homocysteine metabolism in mammals. Semin Thromb Hemost 2000;26:219-25. [Crossref] [PubMed]
- Yan TT, Li Q, Zhang XH, et al. Homocysteine impaired endothelial function through compromised vascular endothelial growth factor/Akt/endothelial nitric oxide synthase signalling. Clin Exp Pharmacol Physiol 2010;37:1071-7. [Crossref] [PubMed]
- Eikelboom JW, Lonn E, Genest J Jr, et al. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann Intern Med 1999;131:363-75. [Crossref] [PubMed]
- Wang XS, Guo LQ, Xiao ZY, et al. Mean platelet volume might be an effective indicator of arterial erectile dysfunction. Asian J Androl 2018;21:62-6. [PubMed]
- Chen Y, Li J, Li T, et al. Association between homocysteine, vitamin B 12, folic acid and erectile dysfunction: a cross-sectional study in China. BMJ Open 2019;9:e023003. [Crossref] [PubMed]
- Xu ZH, Pan D, Liu TY, et al. Effect of prolactin on penile erection: a cross-sectional study. Asian J Androl 2019;21:587-91. [Crossref] [PubMed]
- Yaman O, Tokatli Z, Ozdiler E, et al. Effect of aging on quality of nocturnal erections: evaluation with NPTR testing. Int J Impot Res 2004;16:150-3. [Crossref] [PubMed]
- Caretta N, Palego P, Roverato A, et al. Age-matched cavernous peak systolic velocity: a highly sensitive parameter in the diagnosis of arteriogenic erectile dysfunction. Int J Impot Res 2006;18:306-10. [Crossref] [PubMed]
- Sikka SC, Hellstrom WJ, Brock G, et al. Standardization of vascular assessment of erectile dysfunction: standard operating procedures for duplex ultrasound. J Sex Med 2013;10:120-9. [Crossref] [PubMed]
- Gupta BP, Murad MH, Clifton MM, et al. The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: a systematic review and meta-analysis. Arch Intern Med 2011;171:1797-803. [Crossref] [PubMed]
- Derosa G, Romano D, Tinelli C, et al. Prevalence and associations of erectile dysfunction in a sample of Italian males with type 2 diabetes. Diabetes Res Clin Pract 2015;108:329-35. [Crossref] [PubMed]
- Costa C, Virag R. The endothelial-erectile dysfunction connection: an essential update. J Sex Med 2009;6:2390-404. [Crossref] [PubMed]
- Miner M, Parish SJ, Billups KL, et al. Erectile Dysfunction and Subclinical Cardiovascular Disease. Sex Med Rev 2019;7:455-63. [Crossref] [PubMed]
- Azadzoi KM, Master TA, Siroky MB. Effect of chronic ischemia on constitutive and inducible nitric oxide synthase expression in erectile tissue. J Androl 2004;25:382-8. [Crossref] [PubMed]
- Giovannone R, Busetto GM, Antonini G, et al. Hyperhomocysteinemia as an Early Predictor of Erectile Dysfunction: International Index of Erectile Function (IIEF) and Penile Doppler Ultrasound Correlation With Plasma Levels of Homocysteine. Medicine (Baltimore) 2015;94:e1556. [Crossref] [PubMed]
- Bautista LE, Arenas IA, Peñuela A, et al. Total plasma homocysteine level and risk of cardiovascular disease. J Clin Epidemiol 2002;55:882-7. [Crossref] [PubMed]
- Zhang Z, Xu Z, Dai Y, et al. Elevated serum homocysteine level as an independent risk factor for erectile dysfunction: a prospective pilot case-control study. Andrologia 2017;49. [Crossref] [PubMed]
- Jiang W, Xiong L, Bin Y, et al. Hyperhomocysteinaemia in rats is associated with erectile dysfunction by impairing endothelial nitric oxide synthase activity. Sci Rep 2016;6:26647. [Crossref] [PubMed]
- Endo Y, Iwakawa HO, Tomari Y. Arabidopsis ARGONAUTE7 selects miR390 through multiple checkpoints during RISC assembly. EMBO Rep 2013;14:652-8. [Crossref] [PubMed]
- Maas R, Schwedhelm E, Albsmeier J, et al. The pathophysiology of erectile dysfunction related to endothelial dysfunction and mediators of vascular function. Vasc Med 2002;7:213-25. [Crossref] [PubMed]


