
Carcinogenicity risk associated with tacrolimus use in kidney transplant recipients: a systematic review and meta-analysis
Introduction
Transplantation is currently the therapy of choice for patients with end-stage renal disease (ESRD) as it has been shown to increase survival rates and improve the quality of life (1). Despite the advantages of kidney transplantation, it has been associated with an increased incidence of de novo malignancies, likely due to reduced immunological reactivity as a result of immunosuppressant therapy (2).
Conventional immunosuppressive medications include calcineurin inhibitors (CNI, such as tacrolimus and cyclosporine), azathioprine (Aza), mycophenolate mofetil (MMF), and sirolimus (SRL). Both in vitro and in vivo studies have confirmed that SRL is a mammalian target of rapamycin (mTOR) signaling pathway, with immunosuppressive and anticancer effects (3,4). In addition, Aza has been associated with a higher risk of malignancy compared to other immunosuppressants (5). The current anti-rejection regimen is mainly based on tacrolimus due to the lower incidence of acute rejections compared with cyclosporin A (CsA) (6). However, the risk of malignancies in kidney transplant recipients treated with tacrolimus compared to non-tacrolimus therapy remains controversial. A nested case-control study revealed that kidney transplant recipients who were exposed to a higher tacrolimus concentration were at an increased risk of developing long-term cancers (7). In contrast, a study by Kawahara et al. demonstrated that tacrolimus has a potential to inhibit urothelial tumorigenesis (8). Furthermore, a systematic review in 2005 revealed no statistical difference in tumorigenicity between tacrolimus and CsA (9). The study only compared the carcinogenic risk of tacrolimus to cyclosporine, and the latest study included in the meta-analysis was published in 2002. Since more clinical trials are published, and we also need to compare the carcinogenicity of tacrolimus with other anti-rejection drugs. Therefore, we performed this meta-analysis to more comprehensively assess the carcinogenicity between tacrolimus and non-tacrolimus therapy. We present the following article in accordance with the PRISMA reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-138/rc).
Methods
Search strategy
A systematic literature search was conducted using the Medline (PubMed and Ovid), Embase, Clinical Trials, and Cochrane Library databases with the following search terms: “tacrolimus”, “FK506”, “malignancy”, “tumor”, “cancer”, “neoplasm”, “carcinoma”, “renal transplantation”, and “kidney transplantation” from the inception of the database to May 2021. The complete search strategy is described in Figure 1. All relevant studies were independently verified by two reviewers. All literature were screened by reviewing the titles and abstracts. Studies with combined malignancy as an outcome were excluded, as were review articles and in vitro studies. The full text of the screened eligible studies was comprehensively analyzed. The exclusion criteria are detailed in Figure 1.
Inclusion criteria
The inclusion criteria for studies in this meta-analysis included full texts of clinical trials comparing the tacrolimus group and control group with regards to carcinogenicity in kidney transplant recipients. The primary outcome was the incidence of all types of malignancies, and the secondary outcome was the risk of other specific types of tumors (e.g., skin cancer). Included studies should report at least one outcome, and appropriate assessments were required at the end of the intervention. If different studies included the same population, only the most comprehensive study was selected. Studies were only included in the final meta-analysis if the total number of patients in the cohort and the number of patients who developed malignancies were specified. Exclusion criteria included case reports, reviews, non-clinical studies, editorials, and abstracts.
Data extraction and quality assessment
Data from the final included studies were extracted and recorded in the data extraction form (Table 1). This meta-analysis was performed in accordance with PRISMA guidelines (10). The following data were collated: first author and year of publication, study location, study protocol, number of patients, gender, age at first transplantation, duration of immunosuppression exposure, and follow-up time. Malignancies associated with tacrolimus treatment were documented and subsequently included as a treatment group according to the immunosuppression therapy, such as CsA and mTORs. For the secondary outcome, the incidence of skin cancer was determined in both groups. For clinical studies examining the risk of cancer in tacrolimus users, other information was considered, including the number of patients in the experimental and control groups. Any disagreements or discrepancies were resolved via discussion among the researchers.
Table 1
| Author, publication year | Study location | Study design | Population size | Gender (% male) | Age at first transplant | Exposure | Follow-up time (m) |
|---|---|---|---|---|---|---|---|
| Schena FP, 2009 | America | RCT | 830 | 69.6 | 43.6 | CNI vs. SRL | 24 |
| Kauffman HM, 2005 | America | Retrospective cohort study | 33,249 | 60.3 | – | CNI vs. SRL | 32 |
| Flechner SM, 2011 | Australia | RCT | 469 | 67.7 | 48.9 | TAC + MMF vs. SRL + MMF | 24 |
| Cheung CY, 2009 | China (Hong Kong) | RCT | 76 | 59 | 41 | TAC vs. CsA | 73 |
| Gaber AO, 2008 | America | RCT | 484 | 54.9 | 45.5 | TAC + SRL vs. CsA + SRL | 12 |
| Hardinger KL, 2005 | America | RCT | 200 | 65.3 | 44.7 | TAC vs. CsA | 12 |
| Mahmood MY, 2020 | Iraqi | Retrospective cohort study | 200 | 72 | 36.4 | TAC vs. CsA | 36 |
| Kim J, 2018 | Korea | RCT | 117 | 55.6 | 38.7 | TAC vs. CsA | 120 |
| Krämer BK, 2016 | Germany | RCT | 445 | – | 43 | TAC vs. CsA | 84 |
| Silva HT, 2014 | Brazil | RCT | 456 | >60 | 48.6 | TAC vs. CsA | 48 |
| Krämer BK, 2005 | Germany | RCT | 459 | 67.1 | 43 | TAC vs. CsA | 24 |
RCT, randomized controlled trial; CNI, calcineurin inhibitor; SRL, sirolimus; TAC, tacrolimus; MMF, mycophenolate mofetil; CsA, cyclosporin A.
The quality of the 11 included studies was estimated using the Review Manager software. Risk of bias was assessed in terms of whether there was random sequence generation, allocation concealment, blinding, completeness of results, selective reporting, etc. We scored the quality of each included clinical trials and categorized it as either a high-quality study or a low-quality study.
Statistical analysis
The Review Manager 5.2 software and STATA were used for statistical analyses. We used the Cochrane Q test to assess heterogeneity among the included clinical trials, and the I2 statistic was applied to check the magnitude of heterogeneity. If I2>50%, the results were considered to be significantly heterogeneity. This study was calculated using a fixed effect model and the results are indicative of whether there is heterogeneity between studies. When there was a significant heterogeneity in the results, subgroup analysis was performed to find the sources of heterogeneity. The presence of publication bias between studies was assessed by funnel plots and Begg’s and Egger’s tests. Analyses were performed for all types of malignancies and skin cancer, with risk ratio (RR) and 95% confidence interval (CI) reported for the meta-analysis. A P value of less than 0.05 was considered to be statistically significant.
Results
Study selection
A flow chart detailing the screening and selection of relevant publications is detailed in Figure 1. A search of the database and a manual review of the reference lists of relevant articles resulted in the initial inclusion of 2,261 articles. After an initial screening of titles and abstracts, the full text of the remaining 209 articles was reviewed. A further 198 articles were excluded based on the following: (I) outcomes were not relevant (n=189); or (II) comparisons did not match the study (n=9). Finally, a total of 11 articles were included in this investigation (11-21).
Study characteristics and quality
The 11 included studies were 2 retrospective cohort studies and 9 randomized control trials (RCTs). There were a total of 36,985 kidney transplant recipients (Table 1). A total of 4 studies were conducted in United States, 2 were performed in Germany, and Australia, China, Iraq, Korea, and Brazil each had 1 publication. The average age of the patients at baseline was 43.6 years. Both male and female patients were enrolled in all studies. The average percentage of male patients was 63%. The follow-up time ranged from 12 to 120 months, with a mean of 34.6 months.
Of the 11 included studies, 8 were high quality studies, 1 was assessed as medium quality, and 2 were low quality studies (Figure 2).
The risk of malignancies associated with tacrolimus use
All 11 studies assessed the incidence of malignancies in kidney transplant recipients exposed to tacrolimus. There was no significant heterogeneity among the studies (I2=27%). Meta-analysis showed that the carcinogenic risk was significantly higher in the tacrolimus group compared to the non-tacrolimus group (RR =1.59; 95% CI: 1.19–2.11; P=0.002; Figure 3A).

In 3 studies, the control immunosuppressant was SRL (11-13). The data indicated that the tacrolimus group had a higher risk of carcinogenesis than the SRL group (RR =2.58; 95% CI: 1.62–4.09; P<0.0001), and there was no significant heterogeneity among the studies (I2=7%; Figure 3B). In the other 8 studies, the control immunosuppressant was CsA. The results revealed that there was no significant difference in carcinogenic risk in the tacrolimus group compared with the CsA group (RR =1.12; 95% CI: 0.80–1.56; P=0.52), and there was no significant heterogeneity among studies (I2=0%; Figure 3C). Furthermore, the cohort was then divided into longer and shorter follow-up groups, with a cut-off period of 3 years. In longer follow-up group, the results still demonstrated that there was no significant difference in carcinogenic risk in the tacrolimus group compared with the CsA group (RR =1.08; 95% CI: 0.74–1.59; P=0.69), and there was no significant heterogeneity among studies (I2=0%; Figure 4).
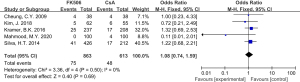
Skin cancer risk associated with tacrolimus use
A total of 5 studies examined the skin cancer risk related to tacrolimus exposure, including 1 retrospective cohort study and 4 RCTs. The control immunosuppressant was CsA in 2 studies and SRL in 3 trials. The results demonstrated that the skin cancer risk was significantly higher in the tacrolimus group compared to the non-tacrolimus group (RR =2.03; 95% CI: 1.25–3.28; P=0.004), and there was no significant heterogeneity among studies (I2=46%; Figure 5).

Publication bias
Publication bias was evaluated by subjective analysis of funnel plots and objective assessment of Begg’s and Egger’s tests. There was no indication of publication bias (P values for Begg’s and Egger’s tests were 1.000 and 0.453, respectively) and the funnel plot was visually symmetrical (Figure 6).
Discussion
In 2005, Webster et al. reported a systemic review which summarized the anti-rejection and adverse effects of tacrolimus compared with cyclosporin in kidney transplant recipients (9). Herein, we updated the research and explored the advantages of tacrolimus, with a particular focus on the risk of tumorigenesis. This report presents an updated meta-analysis assessing the malignancy risk associated with tacrolimus use in renal transplant recipients. According to the literature extraction protocol, 11 papers were finally included. Preliminary results showed that the tacrolimus group had a higher incidence of malignancy compared to non-tacrolimus treatment. Similarly, tacrolimus use was associated with a higher risk of skin cancer compared to the non-tacrolimus treatments. Subgroup analysis include 8 studies sufficiently reported the incidence of malignancy in recipients, and the outcomes showed no differences between tacrolimus and CsA. There were 2 studies conducted by the same author, which considered higher incidence in longer term observation (19,21). In 8 studies, the cohort can be divided into longer and shorter follow-up groups, with a cut-off period of 3 years. There were 5 included literatures with a follow-up period of more than 3 years. In a 10-year follow-up study, an elevated incidence of malignancy was observed with CsA (18). Silva et al. conducted a phase III, open-label, comparative, noninferiority study and demonstrated an increase in the incidence of malignancy in patients treated with tacrolimus compared with those on CsA therapy, using both extended-release and normal preparations (20). Although the tumor incidence tended to increase over the 3-year observation period, there was no significant difference in the malignancy risk between tacrolimus and CsA during the long-term follow-up due to the limited data. Consequently, it was not possible to demonstrate higher cancer tendency in tacrolimus-treated patients, despite a significantly lower incidence of acute rejection.
SRL is a novel class of immunosuppressive drug that targets rapamycin, which is a key serine-threonine kinase regulating cell growth and proliferation. Malignancies were significantly decreased after SRL conversion, which was observed at an early stage in the study and persisted through 24 months (11). Kauffman et al. published a multivariate analysis of posttransplant malignancies in kidney transplant recipients, and suggested that SRL is associated with a significantly decreased risk of posttransplant de novo malignancies and nonskin solid malignancies (12). It is generally believed that low-intensity immunosuppressants can reduce the incidence of cancers. However, others have suggested that SRL-based regimens are not associated with improved cancer risk in kidney transplantation recipients. Nonetheless, a meta-analysis of 3 related papers revealed a lower rate of malignancy associated with SRL treatment compared to tacrolimus group (P=0.006) (22). Najafi and colleagues demonstrated that SRL inhibited the progression of dermal Kaposi’s sarcoma in kidney transplant recipients receiving effective immunosuppressant (23). A review have shown that SRL exerts an antineoplastic effect independent from its immunosuppressive effect (24). Interestingly, a slightly higher rate of infection has been associated with SRL treatment, suggesting that the inhibition of malignancy formation is more consistent with a mTOR-specific effect on tumor biology rather than a reduction in net immunosuppression (25). Conventionally, it is believed that tacrolimus has a more potent effect than SRL, and tacrolimus is the first choice of anti-rejection treatment. Since the positive effect of SRL on malignancies is independent from its low-intensity immunosuppression, it is unlikely that tacrolimus induces tumorigenesis due to its greater immunosuppression. Moreover, in vitro research demonstrated an anti-melanoma effect of tacrolimus, which was partially mediated by inhibiting the oncogenic factor NFAT3 (26). Therefore, the choice of tacrolimus or SRL for kidney transplant recipients cannot be considered solely on the basis of tumorigenic risk.
There were some limitations in this investigation. First, some retrospective studies were included which may have affected the results. Second, the follow-up period in each study was different, and 12 months may be insufficient for evaluating the risk of cancer development. Third, because only English-language databases were searched, the literature search strategy may have language bias. Therefore, more rigorously designed RCT are warranted to confirm the conclusions of this report.
Conclusions
The risk of malignancies associated with tacrolimus use in kidney transplant recipients was analyzed to determine the optimal anti-rejection regimen for such patients. Although the results showed that tacrolimus has a higher risk of carcinogenicity than SRL, it is similar to cyclosporine in terms of carcinogenicity. This conclusion warrants further confirmation with future clinical studies.
Acknowledgments
The authors wish to thank our collaborators for their assistance in this study.
Funding: This project was supported by the Natural Science Foundation of Zhejiang Province (No. LY19H310008).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-138/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-138/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sawada A, Hiragi S, Tamura H, et al. Evaluation of the Quality of Life and Health-Related Quality of Life of Patients With End-Stage Kidney Disease Resulting From Kidney Transplantation Using the Kidney Disease Quality of Life-Short Form and EuroQOL-5 Dimension-5 Level Questionnaires. Transplant Proc 2021;53:881-4. [Crossref] [PubMed]
- Imamura R, Nakazawa S, Yamanaka K, et al. Cumulative cancer incidence and mortality after kidney transplantation in Japan: A long-term multicenter cohort study. Cancer Med 2021;10:2205-15. [Crossref] [PubMed]
- de Fijter JW. Cancer and mTOR Inhibitors in Transplant Recipients. Transplantation 2017;101:45-55. [Crossref] [PubMed]
- Nguyen LS, Vautier M, Allenbach Y, et al. Sirolimus and mTOR Inhibitors: A Review of Side Effects and Specific Management in Solid Organ Transplantation. Drug Saf 2019;42:813-25. [Crossref] [PubMed]
- Jiyad Z, Olsen CM, Burke MT, et al. Azathioprine and Risk of Skin Cancer in Organ Transplant Recipients: Systematic Review and Meta-Analysis. Am J Transplant 2016;16:3490-503. [Crossref] [PubMed]
- Oberbauer R, Bestard O, Furian L, et al. Optimization of tacrolimus in kidney transplantation: New pharmacokinetic perspectives. Transplant Rev (Orlando) 2020;34:100531. [Crossref] [PubMed]
- Lichtenberg S, Rahamimov R, Green H, et al. The incidence of post-transplant cancer among kidney transplant recipients is associated with the level of tacrolimus exposure during the first year after transplantation. Eur J Clin Pharmacol 2017;73:819-26. [Crossref] [PubMed]
- Kawahara T, Kashiwagi E, Li Y, et al. Cyclosporine A and tacrolimus inhibit urothelial tumorigenesis. Mol Carcinog 2016;55:161-9. [Crossref] [PubMed]
- Webster A, Woodroffe RC, Taylor RS, et al. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev 2005;CD003961. [Crossref] [PubMed]
- O'Dea RE, Lagisz M, Jennions MD, et al. Preferred reporting items for systematic reviews and meta-analyses in ecology and evolutionary biology: a PRISMA extension. Biol Rev Camb Philos Soc 2021;96:1695-722. [Crossref] [PubMed]
- Schena FP, Pascoe MD, Alberu J, et al. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation 2009;87:233-42. [Crossref] [PubMed]
- Kauffman HM, Cherikh WS, Cheng Y, et al. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation 2005;80:883-9. [Crossref] [PubMed]
- Flechner SM, Glyda M, Cockfield S, et al. The ORION study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant 2011;11:1633-44. [Crossref] [PubMed]
- Cheung CY, Chan HW, Liu YL, et al. Long-term graft function with tacrolimus and cyclosporine in renal transplantation: paired kidney analysis. Nephrology (Carlton) 2009;14:758-63. [Crossref] [PubMed]
- Gaber AO, Kahan BD, Van Buren C, et al. Comparison of sirolimus plus tacrolimus versus sirolimus plus cyclosporine in high-risk renal allograft recipients: results from an open-label, randomized trial. Transplantation 2008;86:1187-95. [Crossref] [PubMed]
- Hardinger KL, Bohl DL, Schnitzler MA, et al. A randomized, prospective, pharmacoeconomic trial of tacrolimus versus cyclosporine in combination with thymoglobulin in renal transplant recipients. Transplantation 2005;80:41-6. [Crossref] [PubMed]
- Mahmood MY, Rasheed JI, Hussein MR, et al. Comparison of side effect between cyclosporine and tacrolimus as immunosuppressive therapy in Iraqi kidney transplant recipients. Annals of Tropical Medicine and Public Health 2020;23:231-349. [Crossref]
- Kim J, Park J, Hwang S, et al. Ten-year observational follow-up of a randomized trial comparing cyclosporine and tacrolimus therapy combined with steroid withdrawal in living-donor renal transplantation. Clin Transplant 2018;32:e13372. [Crossref] [PubMed]
- Krämer BK, Montagnino G, Krüger B, et al. Efficacy and safety of tacrolimus compared with ciclosporin-A in renal transplantation: 7-year observational results. Transpl Int 2016;29:307-14. [Crossref] [PubMed]
- Silva HT Jr, Yang HC, Meier-Kriesche HU, et al. Long-term follow-up of a phase III clinical trial comparing tacrolimus extended-release/MMF, tacrolimus/MMF, and cyclosporine/MMF in de novo kidney transplant recipients. Transplantation 2014;97:636-41. [Crossref] [PubMed]
- Krämer BK, Montagnino G, Del Castillo D, et al. Efficacy and safety of tacrolimus compared with cyclosporin A microemulsion in renal transplantation: 2 year follow-up results. Nephrol Dial Transplant 2005;20:968-73. [Crossref] [PubMed]
- Knoll GA, Kokolo MB, Mallick R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ 2014;349:g6679. [Crossref] [PubMed]
- Najafi F, Kafaie P, Neamatzadeh H. Treatment of an Early Kaposi's Sarcoma Case Post Kidney Transplantation by Sirolimus: A Case Report. Acta Med Iran 2017;55:139-41. [PubMed]
- Smith A, Niu W, Desai A. The Effect of Conversion from a Calcineurin Inhibitor to Sirolimus on Skin Cancer Reduction in Post-renal Transplantation Patients. Cureus 2017;9:e1564. [PubMed]
- Hahn D, Hodson EM, Hamiwka LA, et al. Target of rapamycin inhibitors (TOR-I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients. Cochrane Database Syst Rev 2019;12:CD004290. [Crossref] [PubMed]
- Xiao T, Chen W, Wang S, et al. Tacrolimus and ascomycin inhibit melanoma cell growth, migration and invasion via targeting nuclear factor of activated T-cell 3. Melanoma Res 2020;30:325-35. [Crossref] [PubMed]
(English Language Editor: J. Teoh)





