
The effects of modified RNA-binding proteins HuR on the biological behavior of the bladder cancer T24 cell line
Introduction
Bladder cancer is one of the most common types of cancers of the urinary system (1). It is estimated that >300,000 patients are diagnosed with bladder cancer every year, and >100,000 die from bladder cancer worldwide (1,2). At present, the main therapy for bladder cancer is a comprehensive treatment mode comprising a combination of surgery, radiotherapy, systemic chemotherapy, and immunity therapy. However, the majority of patients relapse after treatment, and often also suffer from a deterioration in condition (2), and the molecular mechanisms of bladder cancer recurrence and metastasis remain unclear. Although many signaling pathways, such as PI3K/AKT/mTOR, Wnt/β-catenin, and NF-κB star signaling pathways, are involved in the molecular regulation of bladder cancer recurrence and metastasis, due to the complex interaction between different signaling pathways, there is no clear evidence to fully reveal molecular mechanisms of recurrence and metastasis of bladder cancer (3). Thus, further research needs to be conducted on the genetic mechanisms of bladder cancer for therapeutic method development.
Human antigen R (HuR) is a ribonucleic acid (RNA)-binding protein in the embryonic lethal abnormal vision (ELAV) family. In tumors, the role of HuR includes regulating tumor cell proliferation, differentiation, apoptosis, angiogenesis, and lymphangiogenesis (4-7). Previous studies have revealed that the expression of HuR can be detected in ovarian cancer, gastric cancer, breast cancer, cervical cancers, and other tumor tissues, and overexpression HuR in cancer cells has been associated with poor prognosis and resistance to radiotherapy and chemotherapy (4-8). In bladder cancer, high cytoplasmic HuR expression was noticed in cancer tissues, and appeared positively associated with Pathologic TNM stage and grade (9). However, the rare research was published for molecular mechanism of HuR in bladder cancer recurrence and metastasis. Hence, the role and molecular mechanism of HuR in bladder cancer still needs to be further studied and revealed.
Cyclin D1 acts as a regulator of cyclin-dependent kinase (CDKs), and its main function is to promote cell proliferation (10). Among the apoptosis-related proteins, the B-cell lymphoma 2 (BCL-2) family of proteins is the most studied, and is also the most important class of anti-apoptotic protein (11,12). HuR can bind to the adenylate-uridylate-rich element (ARE) fragment of the messenger RNA (mRNA) 3’-untranslated region (3’UTR) of various genes (12). The mRNA encoding the cyclin D1 has been experimentally shown to contain an ARE fragment, and HuR may enhance the stability of the factor mRNA by binding to ARE, and thereby upregulating the expression of the above-mentioned factor protein and exerting the corresponding biological effects. Thus, the present study employed human bladder cancer T24 cells to explore the effects of HuR on the behavior of bladder cancer cells, and examine the relationship between the expression of HuR and cyclin D1 and Bcl-2. We present the following article in accordance with the MDAR reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-123/rc).
Methods
Experimental materials
The T24 human bladder transitional cell carcinoma cell line was purchased from Procell Co., Ltd., and mycoplasma testing was conducted for all cells. We also purchased the following: Roswell Park Memorial Institute (RPMI)-1640 cell culture medium, and trypsin, fetal bovine serum, 1% streptomycin (Gibco; Thermo Fisher Scientific, Inc.); pU6gRNACas9EGFP vector and pIRES2-ZsGreen vector (Addgene, Inc); Lipofectamine 2000TM transfection reagent, TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.); reverse transcription (RT)–polymerase chain reaction (PCR) kits, T4 deoxyribonucleic acid (DNA) ligase, restriction endonuclease Xho I, and EcoR I (Takara Biotechnology, Co., Ltd.); DNA gel recovery kits (Sangon Biotech, Co., Ltd.); Transwell Chamber (Corning, Incorporated); Radio immunoprecipitation assay (RIPA) lysate, bicinchoninic acid (BCA) protein concentration kits, and methylthiazolyldiphenyl-tetrazolium bromide (MTT) test kits (Beyotime, Institute of Biotechnology); apoptosis detection kits (Nanjing KeyGEN BioTECH, Co., Ltd.); HuR antibody, cyclin D1 antibody, and BCL-2 antibody (Abcam); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) horseradish peroxidase (HRP)-labeled goat anti-rabbit secondary antibodies (ProteinTech Group, Inc.).
Cell culture
The T24 cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum and 1% streptomycin, and grown at 37 ℃ and 5% carbon dioxide (CO2). The cells were routinely subcultured when they reached ~80% confluence.
Construction of the HuR overexpression vector
The total RNA of the T24 cells was extracted according to the TRIzol kit’s instructions, complementary DNA (cDNA) was obtained by RT-PCR, and PCR was performed using the following cDNA as a template: Premix Taq 12.5 µL, 1 µL of upstream and downstream primers, 2 µL of template, and ddH2O in 25 µL of reaction system. The thermocycling conditions were 94 ℃ for 5 min, 30 cycles of 94 ℃ for 30 sec, 61 ℃ for 30 sec and 72 ℃ for 2 min, and then a 72 ℃ extension for 10 min. After electrophoresis, the gel was recovered to obtain the HuR gene fragment. The recovered HuR fragment and vector pIRES2-ZsGreen were digested with the restriction endonucleases Xho I and EcoR I, and the digested product was constructed with T4 DNA ligase to construct a HuR expression vector. The constructed HuR overexpression vector was then identified by DNA sequencing.
Construction of the HuR knockdown vector
The guide RNA (gRNA) of the HuR gene (sequence: 5'-AGAGCGATCAACACGCTGAA-3'; synthesized by Shanghai GenePharma Co., Ltd) was designed using the clustered regularly interspaced short palindromic repeats (CRISPR) online design tool (http://crispr.mit.edu/). After annealing to a double strand, the clone was ligated into the pB6gRNACas9EGFP vector digested with Bbs I. After transformation into competent escherichia coli (E. coli) DH5α, the monoclonal extraction plasmid was picked and sequenced to verify that the correctly cloned plasmid was obtained.
Quantitative (q)-PCR to detect the mRNA expression of HuR, cyclin D1, and Bcl-2
After 48 h of transfection, the control, HuR overexpression, and HuR knockdown groups were collected, and the total RNA was extracted according to the TRIzol kit’s instructions. The cDNA was obtained by RT-PCR, and the cDNA was used as a template for quantitative PCR. GAPDH was selected as the internal reference, and the relative expression of HuR, cyclin D1, and Bcl-2 mRNA was determined by the 2–∆∆Cq relative quantitative method. The primer sequences are presented in Table 1. Three independent experiments were repeated.
Table 1
| Name | Primer | Sequence | Size |
|---|---|---|---|
| GAPDH | Forward | 5’-TCAAGAAGGTGGTGAAGCAGG-3’ | 115 bp |
| Reverse | 5’-TCAAAGGTGGAGGAGTGGGT-3’ | ||
| HuR | Forward | 5’-TCATCTACAACCTGGGGCAG-3’ | 162 bp |
| Reverse | 5’-CCATCGCGGCTTCTTCATAG-3’ | ||
| Cyclin D1 | Forward | 5’-CGGACTACAGGGGAGTTTTG-3’ | 273 bp |
| Reverse | 5’-AGGAGGTTGGCATCGGGGT-3’ | ||
| Bcl-2 | Forward | 5’-GCCTTCTTTGAGTTCGGTGG-3’ | 192 bp |
| Reverse | 5’-GAAATCAAACAGAGGCCGCA-3’ |
Western blot of analysis of HuR, cyclin D1, and Bcl-2
The cells of the control, HuR overexpression, and HuR knockdown groups were collected after 48 h. The cell lysate was lysed at 4 ℃, and centrifuged at 12,000 r/min for 30 min, and the supernatant was aspirated to obtain total protein. According to the quantitative results of the BCA assay, 10% sodium dodecyl sulphate-polyacrylamide gel (SDS-PAGE) electrophoresis was conducted for 2.5 h. After the proteins were transferred to the membrane, the negative control membrane was washed in tris-buffered saline (TBS). The membranes were then blocked with 5% skim milk for 1 h at room temperature. Antibodies against HuR, cyclin D1, and Bcl-2 were incubated at 4 ℃ overnight, and the membranes were then washed for 10 min repeatedly for 3 times in TBS with Tween (TBST). The secondary antibodies were incubated for 1 h at room temperature, and then washed with TBST for 10 min 3 times. Next, the membranes were visualized by exposure development. A gray value analysis was performed using BIO-RAD Image Lab software.
MTT assays for cell proliferation activity
The MTT assays were performed 48 h after cell transfection. The control, HuR overexpression, and HuR knockdown groups were seeded in a 96-well plate. A total of 10 µL of MTT was added to each well. After incubating at 37 ℃ for 4 h, the medium was aspirated, and 150 µL of dimethyl sulfoxide was then added, and shaken on a shaker for 10 min. The absorbance value of the optical density of each well at a wavelength of 568 nm was measured with a microplate reader. Each experiment group had three replicate, and three independent experiments were repeated.
Apoptosis detection via Annexin V-APC/7-AAD double staining
The cells of the control, HuR overexpression, and HuR knockdown groups were collected at 48 h after transfection and then washed 2–3 times with pre-cooled phosphate buffered solution (PBS). The cells were adjusted to a cell concentration of 5×105/mL, and resuspended via the addition of 100 µL binding buffer. Next, 5 µL of Annexin V-APC and 5 µL of 7-AAD solution were added and mixed for 10 min at room temperature. Finally, 400 µL of 1X binding buffer was added. The samples were analyzed by flow cytometry. Three independent experiments were repeated.
Transwell assays for cell migration
The cells of the control, HuR overexpression, and HuR knockdown groups were collected 48 h after cell transfection. The cell concentration was diluted to 2×105/mL with serum-free medium. Next, 800 µL of 10% fetal bovine serum containing medium was added to a 24-well plate and placed in a Transwell chamber. Next, 200 µL of each cell suspension was inserted into the Transwell chamber, and cultured at 37 ℃ in a 5% CO2 incubator for 48 h. The Transwell insert was then removed, the chamber was carefully washed with PBS, and the cells were fixed with 70% ice ethanol solution for 1 h. The cells were stained with 0.5% crystal violet staining solution, left at room temperature for 20 min, washed with PBS, and the unmigrated cells on the upper chamber were then wiped clean with a clean cotton ball. Pictures were taken under a microscope, and the number of cells was counted. Three independent experiments were repeated.
Statistical analysis
All the experiments were repeated at least 3 times. The statistical analysis was performed using SPSS 23.0 statistical software (SPSS, Inc.) The data are expressed as the mean ± standard deviation, and an analysis of variance and a post-hoc test for multiple comparisons were used for comparisons between groups. A P value<0.05 indicated a statistically significant difference.
Results
Construction of the HuR overexpression vector and knockdown vectors
As Figure 1A,1B show, the constructed HuR overexpression and knockdown vectors were sequenced and analyzed, which indicated that the inserted sequence and site were correct, and the overexpression of the target gene HuR and the knockdown vector plasmid had been successfully constructed.
Silencing and overexpression of HuR in response to HuR transfection and HuR knockdown by CRISPR-associated proteins 9 (cas9)
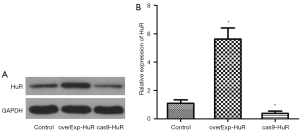
The protein expression levels of HuR were detected by western blotting, and are set out in Figure 2A. Compared to the control group, the expression level of HuR was significantly increased in the HuR overexpression group, and significantly decreased in the HuR knockdown group (P<0.01). The relative expression levels of mRNA of HuR were detected by PCR (see Figure 2B). Compared to the control group, the expression level of HuR was significantly increased in the HuR overexpression group, and significantly decreased in the HuR knockdown group (P<0.01).
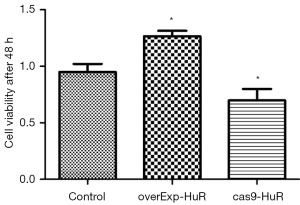
HuR promotes the proliferation of T24 cells
Compared to the control group, after 48 h, the cell viability was significantly increased in the HuR overexpression cells, and significantly decreased in the HuR knockdown group (P<0.01; see Figure 3).
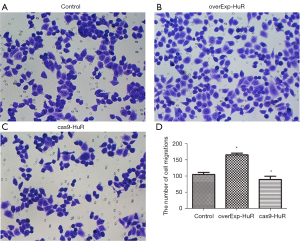
HuR promotes the migration of T24 cells
Compared to the control group, the number of migrated cells was significantly increased in the HuR overexpression group, and significantly decreased in the HuR knockdown group (P<0.01; see Figure 4).
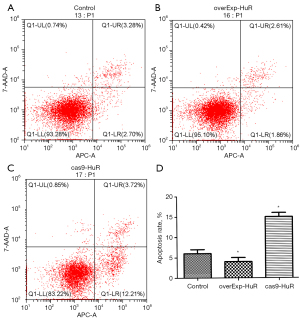
HuR inhibits T24 cell apoptosis
Compared to the control group, the apoptotic rate was significantly decreased in the HuR overexpression group, and significantly increased in the knockdown group (P<0.01; see Figure 5).
Effect of HuR on the expression of cyclin D1 and Bcl-2
The expression levels of the proteins of cyclin D1 and Bcl-2 were detected by western blotting (see Figure 6A). Compared to the control group, the expression levels of cyclin D1 (P<0.01) and Bcl-2 (P<0.01) were significantly increased in the HuR overexpression group, and significantly decreased in the HuR knockdown group.
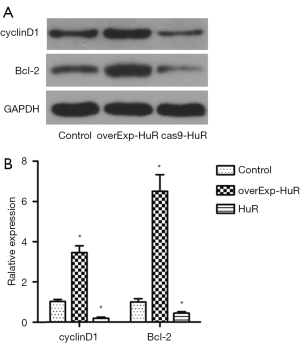
The relative expression levels of mRNA of cyclin D1 and Bcl-2 were detected by PCR (see Figure 6B). Compared to the control group, the expression levels of cyclin D1 (P<0.01) and Bcl-2 (P<0.01) were significantly increased in the HuR overexpression group, and significantly decreased in the HuR knockdown group.
Discussion
The progression of tumors is the result of a series of interactions between multiple genes in which tumor gene expression and function regulation have altered levels that can be regulated at the pre-transcriptional, transcriptional, and post-transcriptional levels (13-15). At the post-transcriptional level, there is a class of proteins that regulate the metabolic processes of RNA and bind to RNA, which affects the metabolism of RNA. These proteins are called RNA-binding proteins (RBPs), of which HuR is a widely studied type (16-18). HuR is a member of the ELAV family of RNA-binding proteins, located at 19p13.2, which encodes a variety of proto-oncoproteins (16-18). HuR binds to and stabilizes a partial fragment in the 3'UTR, which is widely expressed in the cytoplasm and nucleus of mammalian cells, and is mainly expressed in the nucleus in normal tissue cells. In tumor cells, there is a positive increase in HuR expression, especially in the cytosol (19,20). Young et al. reported that HuR was significantly more highly expressed in colon tumor tissues than normal colon tissues, and the increased expression of HuR promoted the development of colon cancer (21). Heinonen et al. demonstrated that in breast cancer patients with the non-breast cancer susceptibility gene 1/2 mutation, the positive rate of cytoplasmic HuR was 39.4%, and the high expression of HuR was closely associated with the number of lymph node metastases (22,23). The experimental results also suggested that HuR may be involved in lymph node metastasis (22,23). A recent study revealed that HuR was highly expressed in various tumor tissues, such as breast, gastric, esophageal, and ovarian cancer tissues (24). In the present study, the proliferation rate and migratory ability of HuR overexpression cells were significantly increased, and the apoptotic rate was decreased. The proliferation rate and migratory ability of HuR knockdown cells were significantly decreased, and the apoptotic rate was significantly increased. Thus, HuR was shown to promote the proliferation and migration and decrease the apoptosis of human bladder cancer T24 cells.
The cyclin D1 gene is a proto-oncogene that plays a very important regulatory role in the cell cycle of eukaryotic cells (25,26). Cyclin D1 binds and activates cyclin dependent kinase 4 (CDK4), which is a characteristic of the G1 phase. The G1 cycle inhibitory protein [retinoblastoma protein (Rb)] is phosphorylated, and the phosphorylated Rb protein is then cleaved from its bound early 2 factor (E2F) transcription factor; in turn, the E2F transcription factor initiates the transcription of the cell-cycle gene and forms a protein complex with a cell cycle-dependent kinase, and thus, the cells pass through the cell cycle G1/S control point and enter the S phase (25,26). The expression of cyclin D1 has been shown to be significantly increased in many tumor tissues, such as breast cancer, nasopharyngeal carcinoma, lung cancer, and liver cancer tissues (27-30).
In the present study, the expression of cyclin D1 was significantly increased in the HuR overexpression group, and significantly decreased in the HuR knockdown group, which suggests that the mechanism of HuR on the biological behavior of T24 cells may be related to the expression of cyclin D1 and the cell cycle. The Bcl-2 gene is also a proto-oncogene that alters the permeability of the mitochondrial outer membrane and inhibits the release of cytochrome C into the cytoplasm, thereby preventing the activation of the caspase cascade and the inhibition of apoptosis. The level of Bcl-2 protein expression regulates apoptosis. When Bcl-2 expression is high, apoptosis is inhibited. Conversely, when Bcl-2 expression is low, apoptosis is promoted. The present study revealed that compared to the control group, the expression of Bcl-2 was significantly increased in the HuR overexpression group, and the expression of Bcl-2 was significantly decreased in the HuR knockdown group, which suggests that HuR inhibits the apoptosis of human bladder cancer T24 cells, which is consistent with the conclusion that HuR inhibits apoptosis. It is indicated that HuR plays a key role in the occurrence and development of bladder cancer and may serve as a potential target for the development of new anti-bladder cancer drugs.
Conclusions
In conclusion, the RNA-binding protein HuR promotes the proliferation and migration of bladder cancer T24 cells, and inhibits apoptosis, and the mechanism may be related to its effect on the cell cycle and apoptosis. The expression of HuR is related to cyclin D1 and the apoptosis-related protein Bcl-2. Thus, the HuR gene may be a novel target gene for targeted therapy in bladder cancer patients. However, this study had some limitations. First, only one bladder cancer cell line was examined in this study. Second, experiments of both animal and human tissue samples were not conducted. Third, due to a lack of finance, only 1 apoptosis-related protein was selected for this experiment. Thus, further research, especially in-vivo studies, need to be conducted in the future.
Acknowledgments
Funding: This work was supported by the Wenzhou Public Welfare Science and Technology Foundation (No. Y20160336); the Zhejiang Province Natural Sciences Foundation (No. LQ17H050002); the Natural Science Foundation of Inner Mongolia Autonomous Region of China (Nos. 2017BS0805, 2018BS08014, and 2021MS08009); the Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (No. NJYT-20-B22); the Research Program of Science and Technology at Universities of Inner Mongolia Autonomous Region (Nos. NJZZ18186, NJZY22049, and NJZZ22063); the Research Funds of Baotou Medical College (Nos. BYJJ-YF 201616, BSJJ201707, and BSJJ201708); the Nanshan District (Shenzhen) Education (Health) Science and Technology Project (No. 2020009); and the Baotou Medical College Postgraduate Research and Innovation Project (No. bycx2021002).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-123/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-123/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-123/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 2013;63:234-41. [Crossref] [PubMed]
- Chestnut C, Subramaniam D, Dandawate P, et al. Targeting major signaling pathways of bladder cancer with phytochemicals: a review. Nutr Cancer. 2021;73:2249-71. [Crossref] [PubMed]
- Dong R, Lu JG, Wang Q, et al. Stabilization of Snail by HuR in the process of hydrogen peroxide induced cell migration. Biochem Biophys Res Commun 2007;356:318-21. [Crossref] [PubMed]
- Kang MJ, Ryu BK, Lee MG, et al. NF-kappaB activates transcription of the RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric tumorigenesis. Gastroenterology 2008;135:2030-42, 2042.e1-3.
- Huang YH, Peng W, Furuuchi N, et al. Delivery of Therapeutics Targeting the mRNA-Binding Protein HuR Using 3DNA Nanocarriers Suppresses Ovarian Tumor Growth. Cancer Res 2016;76:1549-59. [Crossref] [PubMed]
- Cho NH, Kang S, Hong S, et al. Elevation of cyclin B1, active cdc2, and HuR in cervical neoplasia with human papillomavirus type 18 infection. Cancer Lett 2006;232:170-8. [Crossref] [PubMed]
- Mehta M, Basalingappa K, Griffith JN, et al. HuR silencing elicits oxidative stress and DNA damage and sensitizes human triple-negative breast cancer cells to radiotherapy. Oncotarget. 2016;7:64820-35. [Crossref] [PubMed]
- Miyata Y, Watanabe S-I, Sagara Y, et al. High expression of HuR in cytoplasm, but not nuclei, is associated with malignant aggressiveness and prognosis in bladder cancer. PLoS ONE. 2013;8:e59095. [Crossref] [PubMed]
- Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med (Berl) 2016;94:1313-26. [Crossref] [PubMed]
- Majtnerová P, Roušar T. An overview of apoptosis assays detecting DNA fragmentation. Mol Biol Rep 2018;45:1469-78. [Crossref] [PubMed]
- Rathore R, McCallum JE, Varghese E, et al. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 2017;22:898-919. [Crossref] [PubMed]
- Deng N, Zhou H, Fan H. Single nucleotide polymorphisms and cancer susceptibility. The Molecular Basis of Human Cancer. Springer New York, 2017.
- James E, Jenkins TG. Epigenetics, infertility, and cancer: future directions. Fertil Steril 2018;109:27-32. [Crossref] [PubMed]
- Rybstein MD, Bravo-San Pedro JM, Kroemer G, et al. The autophagic network and cancer. Nat Cell Biol 2018;20:243-51. [Crossref] [PubMed]
- DeMicco A, Naradikian MS, Sindhava VJ, et al. B Cell-Intrinsic Expression of the HuR RNA-Binding Protein Is Required for the T Cell-Dependent Immune Response In Vivo. J Immunol 2015;195:3449-62. [Crossref] [PubMed]
- Peng SS, Chen CY, Xu N, et al. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J 1998;17:3461-70. [Crossref] [PubMed]
- Mukherjee N, Corcoran DL, Nusbaum JD, et al. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol Cell 2011;43:327-39. [Crossref] [PubMed]
- Kim I, Kwak H, Lee HK, et al. β-Catenin recognizes a specific RNA motif in the cyclooxygenase-2 mRNA 3'-UTR and interacts with HuR in colon cancer cells. Nucleic Acids Res 2012;40:6863-72. [Crossref] [PubMed]
- Sun DQ, Wang Y, Liu DG. Cancer cell growth suppression by a 62nt AU-rich RNA from C/EBPβ 3'UTR through competitive binding with HuR. Biochem Biophys Res Commun 2012;426:122-8. [Crossref] [PubMed]
- Young LE, Sanduja S, Bemis-Standoli K, et al. The mRNA binding proteins HuR and tristetraprolin regulate cyclooxygenase 2 expression during colon carcinogenesis. Gastroenterology 2009;136:1669-79. [Crossref] [PubMed]
- Heinonen M, Hemmes A, Salmenkivi K, et al. Role of RNA binding protein HuR in ductal carcinoma in situ of the breast. J Pathol 2011;224:529-39. [Crossref] [PubMed]
- Heinonen M, Fagerholm R, Aaltonen K, et al. Prognostic role of HuR in hereditary breast cancer. Clin Cancer Res 2007;13:6959-63. [Crossref] [PubMed]
- Danilin S, Sourbier C, Thomas L, et al. Role of the RNA-binding protein HuR in human renal cell carcinoma. Carcinogenesis 2010;31:1018-26. [Crossref] [PubMed]
- Casimiro MC, Di Sante G, Di Rocco A, et al. Cyclin D1 Restrains Oncogene-Induced Autophagy by Regulating the AMPK-LKB1 Signaling Axis. Cancer Res 2017;77:3391-405. [Crossref] [PubMed]
- Ju X, Jiao X, Ertel A, et al. v-Src Oncogene Induces Trop2 Proteolytic Activation via Cyclin D1. Cancer Res 2016;76:6723-34. [Crossref] [PubMed]
- Bartkova J, Lukas J, Müller H, et al. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer 1994;57:353-61. [Crossref] [PubMed]
- Hong JI, Xia HE, Cheng SQ. The Expression of p16 and Cyclin D1 in Nasopharyngeal Carcinoma and its Clinical Significance. Journal of Oncology 2010;16:370-3.
- Pandey A, Bahl C, Sharma S, et al. Functional role of CyclinD1 polymorphism (G870A) in modifying susceptibility and overall survival of North Indian lung cancer patients. Tumori 2018;104:179-87. [Crossref] [PubMed]
- Huang CZ, Huang WZ, Zhang G, et al. In vivo study on the effects of curcumin on the expression profiles of anti-tumour genes (VEGF, CyclinD1 and CDK4) in liver of rats injected with DEN. Mol Biol Rep 2013;40:5825-31. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


