
Effect of Huaji Jianpi Decoction on the semen quality of high-fat diet-induced obese mice
Introduction
The obesity rate has risen in recent years, and more than 10% of adults worldwide meet the obesity criteria (1). Obesity has become a global health problem. According to statistics, 1 in 25 obese adult men suffer from male infertility. Moreover, the incidence of infertility in obese and overweight men is ever increasing. Obesity leading to reduced male fertility has become a hot issue in current research. Recently, scholars have proposed that obesity reduces male fertility by affecting spermatogenesis and semen quality (2,3), mainly through changes in testicular weight, spermatogenic tubule diameter, and number of spermatogenic cells, as well influencing semen parameters (such as the decrease of sperm count, density, and viability) (4). Currently, the treatment of male hypogonadism is mostly through western medicine, but long-term use exhibits serious side effects. Therefore, traditional Chinese medicine (TCM), with low side effects and stable efficacy, is considered to be a more effective alternative treatment option. Nevertheless, the process of TCM intervention involves various components, pathways, and targets, and the interaction network of corresponding functional protein targets is complex (5). Huaji Jianpi Decoction is a kind of TCM made with 14 herb ingredients which can be used to invigorating spleen for eliminating dampness according to ancient Chinese prescriptions.
At present, no unified criteria for evaluating TCM treatment of obesity-induced reproductive impairment has been established. In this study, by applying the high-fat diet-induced obese mice model, whether Huaji Jianpi Decoction could lower lipids to improve the semen quality of obese male mice was investigated, and the effect of Huaji Jianpi Decoction on the treatment of obesity-induced low fertility was explored, with an aim to provide basic information for the treatment of obesity and obesity-induced low male fertility by TCM. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-115/rc).
Methods
Ethical statement
All animal research was approved by the Ethics Committee of Hebei University (approval ID: IACUC-2021XS038). The animal research was performed in compliance with the Hebei University Laboratory Animal Welfare and Ethics guidelines of the Animal Welfare and Ethical Committee of Hebei University.
Huaji Jianpi Decoction preparation
The Huaji Jianpi Decoction included stir-fried Atractylodes (30 g), stir-fried Citrus aurantium (15 g), stir-fried Atractylodes Atractylodes (30 g), Poria cocos (15 g), French Pinellia (10 g), tangerine peel (10 g), Codonopsis (15 g), Jiao Hawthorn (15 g), Jiao Shenqu (15 g), Jiao Malt (15 g), stir-fried Coix seed (30 g), fried Alisma (15 g), lotus leaf (10 g), and roasted licorice (6 g). First, all medicinal materials were mixed using a Waring blender, soaked in double-distilled water (50 times the medicinal materials) for 2 h, and boiled for 1 h. After cooling, the decoction was filtered through 2 layers of cotton gauze. The filtrates obtained from 3 cycles of the procedure were combined. After filtration, the solution was concentrated into a residue in a vacuum evaporator and distilled into liquid. The yield of the aqueous extract was 3.85 g/mL and the volume was 60 mL (based on the original amounts of the herbal ingredients). After preparation, the reagents were put into glass bottles and stored at 4 ℃.
Animal models
A total of 100 specific pathogen-free (SPF)-grade healthy 3-week C57BL/6J male mice weighed about 15 g (The Charles River Laboratories, Beijing, China) were adaptively bred for 1 week and randomly allocated into the normal group (30 mice on a normal diet) and the model group (70 mice on a high-fat diet). Food intake and water consumption were recorded daily. After 10 weeks of high-fat diet exposure, the obese mice were screened by weighing mice and calculating Lee’s index. Obesity was defined as a 20% increase in weight compared with the normal group. Moreover, micro-CT was applied to further confirm the total volume of the adipose tissue. The high-fat feed ingredient was D12492 (Research Diets, Inc.; New Brunswick, USA), including 20 kcal% protein, 20 kcal% carbohydrate, and 60 kcal% fat. The ordinary feed was purchased from Weitonglihua (Beijing Weitong Lihua Laboratory Animal Technology Co., LTD.; No. 1184, Baishan Village, Baishan Town, Changping District, Beijing, China).
Drug intervention
A total of 50 obese model mice were selected and randomly allocated into the model blank group, orlistat group [0.091 g/(kg·d) dose], low-dose TCM group [17.52 g/(kg·d) dose], medium-dose TCM group [35.04 g/(kg·d) dose], and high-dose TCM group [70.07 g/(kg·d) dose]. The dose of Huaji Jianpi Decoction was determined by converting the dose of human in to mice according to the body surface area. Mice in the drug experimental groups were given corresponding drugs, and mice in the normal group and the model blank group were given corresponding volumes of distilled water by oral gavage at 08:00 every day for 6 weeks. The dose was adjusted according to the weekly weight changes of mice. During the administration period, mice in the drug experimental group were still fed with a high-fat diet. Drugs were administered by gavage after 20 min in a water bath at 37 ℃. After administration, the drugs were stored at 4 ℃.
Testis coefficient evaluation in mice
After 6-week intragastric administration, the mice were sacrificed by the cervical dislocation method 2 h after the last administration. Subsequently, the mice were fixed, and their abdomens were sprayed using 75% alcohol. After disinfection, the abdominal cavity of the mice was quickly opened. Both testicles were taken, around which the fatty tissue was cleaned up. An electronic balance was used for weighing, and the wet testis weight of the mice was measured to calculate the testis coefficient. The testis coefficient was defined using the following formula: testis coefficient = (testis weight/mice weight) × 100%. The tissue was stored in a freezer at −80 ℃ for subsequent use.
Semen detection in mice
After intragastric administration for 6 weeks, the mouse epididymal tail tissue was obtained. The removed epididymal tail was washed twice in PBS solution and placed in 0.9% NaCl solution. The semen was squeezed out using tweezers under a stereo microscope (Olympus, Japan), followed by 30 min incubation in an incubator. After full dissociation, the sperm was placed on a counting plate (pre-placed on a 37 ℃ constant temperature plate in advance). The sperm suspension incubated in the 37 ℃ cell incubator (Thermo Scientific, USA) was taken out and mixed thoroughly. Next, 10 µL of semen was dropped on the sperm counting plate. The glass slide was covered and placed on the thermostatic plate. More than 200 sperm were observed and counted under the microscope. The sperm quality analysis system was used to detect the sperm indicators.
Transmission electron microscope (TEM) observation
The mouse testis tissues were placed on ice cubes and quickly cut into 1 mm3 pieces. Subsequently, mouse testis tissues were fixed in 2.5% glutaraldehyde solution for 2 h, washed 3 times with phosphate buffer saline (PBS) (0.1 mol/L), and added with 1% osmium acid for 1 h fixation. After 3 washes with PBS (0.1 mol/L), the tissues were dehydrated using gradient alcohol (30%, 50%, 70%, 85%, 95%, and 100%) and acetone, and embedded in Epon 812. The semi-thin sections (0.5–2 nm) were stained with toluidine blue to observe mouse testis morphological changes under an optical microscope. After ultrathin sectioning, copper netting, and lead citrate and uranyl acetate staining, testis ultrastructure was observed using a TEM (Japan Electronics Corporation, Japan).
Reverse-transcription quantitative PCR (RT-qPCR)
Total RNA from mouse testis tissues was extracted using TRIpure Reagent Total RNA Extraction Reagent (Beijing Adler Biotechnology Co., Ltd., China) as per the manufacturer’s instructions. RT-qPCR was performed for expression level analysis using the 5× HiScript II Select qRT SuperMix II and AceQ qPCR SYBR Green Master Mix (Vazyme, China) with the 7300 Real-Time PCR System (Applied Biosystems, USA). The data were normalized to β-actin expression and then further normalized to the negative control unless otherwise indicated. Custom primers for C/EBP-α, PPAR-γ, tumour necrosis factor α (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) were synthesized as follows: β-actin forward primer 5'-ACTCATCGTACTCCTGCTTGCTGA-3' and reverse primer 5'-AGGGAAATCGTGGGTGACATCAAA-3'; C/EBP-α forward primer 5'-CCTTCAACGACGAGTTCCTG-3' and reverse primer 5'-TGGCCTTCTCCTGCTGTC-3'; PPAR-γ forward primer 5'-CTGGCCTCCCTGATGAATAA-3' and reverse primer 5'-GGCGGTCTCCACTGAGAATA-3'; TNF-α forward primer 5'-CAGATTGACCTCAGCGCTGAGTTG-3' and reverse primer 5'-ACCCTCACACTCAGATCATCTTCT-3'; MCP-1 forward primer 5'-GGGATCATCTTGCTGGTGAA-3' and reverse primer 5'-AGGTCCCTGTCATGCTTCTG-3'. The three-step method was used. Data were analyzed using the 2−ΔΔCt method.
Statistical analysis
All data were statistically analyzed using SPSS 19.0 software. The measurement data were represented as mean ± standard deviation (). Comparisons between groups were analyzed using one-way analysis of variance, and comparisons among multiple groups were analyzed using the LSD method. The difference was statistically significant when P<0.05.
Results
Huaji Jianpi Decoction could improve the semen quality of obese mice
As indicated by the results of the animal model, obese mice showed a much lower testicular wet weight and testicular coefficient than normal mice (ND) (P<0.05). Sperm density, sperm motility, sperm forward motion force (PR), and total sperm motility (PR + NP) of mice in the model blank group were all lower than those in the normal group (P<0.05).
The testicular wet weight and testicular coefficient were strongly associated with semen quality. Compared with mice in the control group (HFD), the testicular wet weight and testicular coefficient of mice with drug administration were increased to varying degrees (P<0.05). No difference in testicular wet weight was found between the low-dose (HFD + HLG) group and the medium-dose (HFD + HMG) group (Figure 1A). However, the testicular wet weight in the remaining groups showed differences, with the high-dose group (HFD + HHG) showing more obviously increased testicular wet weight (P<0.05). There was no difference in testicular coefficient between the orlistat group (HFD + orlistat) and the Huaji Jianpi Decoction group (P>0.05) (Figure 1B).

Based on the results in this study, the sperm density of mice with drug administration was increased to varying degrees relative to those in the HFD group (P<0.05) (Figure 2A). The sperm density of mice in the HFD + HHG, HFD + HMG, and HFD + HLG groups was higher than that in the HFD + orlistat group (P<0.05), while compare with other groups, only the difference was observed between the Huaji Jianpi Decoction groups, HFD + HMG, and HFD + HHG groups. Mice in the HFD + HHG group showed more obvious sperm density improvement (P<0.05) (Figure 2B). The sperm motility of the HFD + orlistat group showed no difference to that of the HFD group (P>0.05), but the Huaji Jianpi Decoction groups exhibited much higher sperm motility than that of the HFD group (P<0.05). No difference among the Huaji Jianpi Decoction groups was found (P>0.05). Compared with the ND group, the PR of the drug administration groups was improved to varying degrees. In addition to the HFD + orlistat group and the HFD + HMG group (P>0.05), the drug groups showed different PR. The HFD + HHG group exhibited more obvious effects (P<0.05) (Figure 2C). The PR + NP of the HFD + orlistat, HFD + HMG, and HFD + HHG groups was notably higher than that in the HFD group (P<0.05). The PR + NP of the HFD + orlistat group was different from that of the HFD + HMG and HFD + HHG groups (P<0.05), and the HFD + HHG group showed a more significant increase in PR + NP (P<0.05) (Figure 2D).
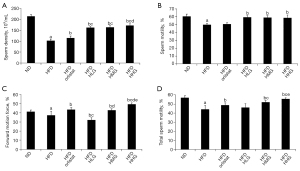
In summary, Huaji Jianpi Decoction administration caused increased testicular wet weight, testicular coefficient, and semen parameters. Huaji Jianpi Decoction could also improve testicular weight, sperm density, sperm motility, forward motility, and total sperm motility.
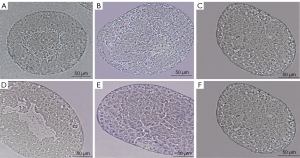
Huaji Jianpi Decoction could reduce the Morphological structure of spermatogenic cells and seminiferous tubules in obese mice
As shown in Figure 3, the male mice of each group showed the complete basic structure of the seminiferous tubules with no obvious tissue damage. The substrate membrane of the seminiferous tubule (whose tube cavity has 5–8 layers of cells) was relatively complete. All levels of sperm cells closely connected to supporting cells were clear in all groups except for the HFD group. The HFD group exhibited decreased cell layers in the seminiferous tubule, accompanied by ambiguous and disordered structure of sperm cells at all levels, and supporting cells are lost to apoptosis (Figure 3B).
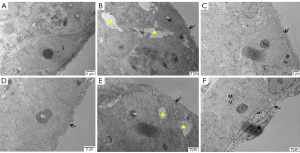
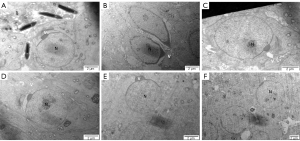
As shown in Figure 4, the seminiferous tubules surrounded by Myoid cell substrate membrane and connected to Sertoli cells and spermatogenic cells were thin and regular in the ND (Figure 4A), HFD + orlistat, HFD + HLG, HFD + HMG, and HFD + HHG groups (Figure 4C-4F). Compared with the ND group, the HFD (Figure 4B) group showed more obvious microstructural changes (severely damaged, thickened, and irregular substrate film). Meanwhile, a wide range of cell gaps was observed in the luminal epithelial cells, which caused cellular separation and destruction. Large areas where cytoplasm was not present were found in luminal epithelial cells, showing swollen mitochondria and cytoplasm with empty bubbles.
Compared with the ND group (Figure 5A), a wide space around the nucleus and dramatically decreased mitochondrial numbers were observed in the HFD group (Figure 5B). According to our results, mitochondria were observed in the cytoplasm of spermatogonia with an obvious nucleolus surrounded by chromosome clumping (as shown by the black arrow) in the ND, HFD + orlistat, HFD + HLG, HFD + HMG, and HFD + HHG groups (Figure 5C-5F). No other obvious differences were observed in addition to these differences.
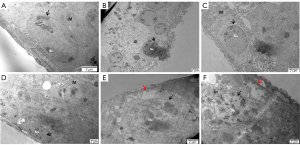
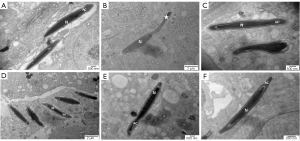
The ultrastructure of rounded sperm cells showed similar results to that of spermatogonia. Compared with the ND group (Figure 6A), in the HFD group (Figure 6B), early sperm cells exhibited malformation and an area without cytoplasm due to the lack of an acrosome cap. Early sperm cells whose proximal part was covered by an acrosome cap presented as a circle in the ND HFD + orlistat, HFD + HLG, HFD + HMG, and HFD + HHG groups (Figure 6C-6F).
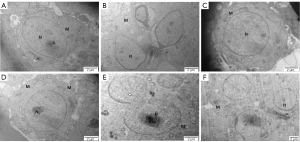
According to the results of the ultrastructure of rounded sperm cells, compared with the HFD group, the proximal part of the round nucleus of early sperm cells was covered by a vesicle, and small empty bubbles were observed in the cytoplasm of other groups (Figure 7).
The head of long-form sperm cells showed an abnormal acrosome in the HFD group (as shown by the asterisk), while the sperm nucleus was normal. Compared with that in the ND group, no difference in the structure of long-form sperm cells was found in the HFD + orlistat, HFD + HLG, HFD + HMG, and HFD + HHG groups (Figure 8).
Huaji Jianpi Decoction downregulated the expression of TNF-α and MCP-1
The expression of TNF-α and MCP-1 was improved in the HFD group (P<0.05) compared with the ND group. No difference in MCP-1 expression was observed between the HFD and HFD + HLG groups (P>0.05). The expression of TNF-α and MCP-1 was decreased to varying degrees in the HFD + orlistat, HFD + HMG, and HFD+HHG groups (P<0.05). No difference was found in TNF-α mRNA expression between the HFD + orlistat and HFD + HLG groups, and the HFD + HMG and HFD + HHG groups (P>0.05). Nevertheless, there was a difference in the expression of TNF-α mRNA between the drug administration groups, with the HFD + HHG group showing a more obvious reduction (P<0.05). There was no difference in the expression of MCP-1 mRNA in the HFD + orlistat and HFD + HHG groups (P>0.05). Most obviously, the expression of TNF-α and MCP-1 mRNA was reduced in the HFD + HHG group (Figure 9).

Discussion
This study provided evidence that Huaji Jianpi Decoction could decrease the body fat and blood lipid levels of obese mice, increase the testicular wet weight and testis coefficient, and enhance semen parameters. Different doses of Huaji Jianpi Decoction could improve testicular weight, sperm density, sperm motility, forward motility, and total sperm motility to varying degrees, downregulate TNF-α and MCP-1 expression, inhibit germ cell apoptosis, and improve semen quality. Recent study has demonstrated that obesity is a chronic metabolic disease, which leads to multiple complications due to excessive fat accumulation or abnormal distribution in the body as a result of the body’s energy intake exceeding consumption (6). A previous study proposed that obesity exerts negative effects on sperm motility and semen parameters, and is a high-risk factor for male infertility (7). It has been shown that the fertility of obese male mice was improved following lipid lowering and weight loss by vertical banding gastric decongestion (8). After Huaji Jianpi Decoction administration into obese male mice for 6 weeks, the body fat content and blood lipid levels were reduced, the testicular wet weight and semen content were increased, and semen parameters were improved. The above results suggest that Huaji Jianpi Decoction can improve semen quality in obese mice through weight loss and lipid reduction.
Growing studies have revealed that increased obesity rates coincide with decreased sperm quality and increased male infertility (9,10), suggesting that obesity is associated with male fertility (11). After 4 weeks of the high-fat diet, mouse sperm concentration and sperm motility were reduced. Previous study also demonstrated that sperm mitochondria were impaired, ATP was reduced, and reactive oxygen species (ROS) were elevated (12). Moreover, total sperm count was markedly lower in obese males than in males with normal weight (13). Obese individuals have lower sperm mitochondrial activity, as well as higher ROS and DNA fragmentation levels (14). Our results showed that sperm density, viability, sperm forward motility, and total sperm viability were significantly reduced in obese mice relative to those in ND. These results could be attributed to the mitochondrial aerobic respiratory response and the uncoupling of ATP, suggesting that obesity not only increases the risk of sperm DNA breakage, but is also associated with reduced mitochondrial activity. Furthermore, apoptosis is crucial for the development of male germ cells, and excessive apoptosis is present in the sperm of both infertile patients and infertile mice (15). High-fat foods induce apoptosis in murine germ cells, which then promotes infertility (16). According to the results in this study, the testicular wet weight and testis coefficient were significantly reduced in the model blank group. Reduced mitochondria in the testicular ultrastructure and the apoptosis of supporting cells and germ cells in the lumen of the germinal ducts were observed, which is possibly associated with obesity-induced apoptosis of spermatogonia, spermatocytes, and Sertoli cells (17). Spermatogenesis, as a complex process, relies on coordinated cell proliferation and apoptosis, and excessive apoptosis can lead to sperm abnormalities (18). Therefore, inhibiting excessive apoptosis and re-establishing the balance between cell proliferation and apoptosis may be effective means for treating abnormal spermatozoa. It was hypothesized that Huaji Jianpi Decoction could improve the reproductive function of mice by protecting the mitochondrial morphology of testes and inhibiting the excessive apoptosis of spermatogenic cells.
Our results showed that fat volume and distribution in normal and obese mice were significantly higher than in ND. Fatty acids can synthesize and secrete a variety of adipokines that can regulate reproductive function through the neuroendocrine system or by acting directly on the testis. However, obesity can lead to adipocyte dysfunction and cause a systemic inflammatory response (19), which can be characterized by abnormal secretion of pro-inflammatory cytokines (TNF-α and MCP-1) and the activation of inflammatory pathways (20). These pathways can act on the testis indirectly or directly and affect the functioning of the reproductive system. TNF-α induces Fas upregulation through NF-κB activation in mouse support cells. Fas upregulation initiates apoptotic pathways and triggers apoptosis in testis cells, thereby causing damage to the blood-testis barrier (21). In particular, Fas activates the release of Cytc and increases the content of intercellular membrane protein Cytc, which further activates apoptosis-related factors (such as caspase-3) and increases the content of Cytc (22).
To investigate the effects of Huaji Jianpi Decoction on inflammatory factors and apoptosis, we used RT-qPCR to analyze TNF-α and MCP-1 mRNA expression. It was found that the expression levels of TNF-α and MCP-1 in the testicular tissues of mice in the model blank group were significantly higher than those of mice in the normal group. Obesity may stimulate the recruitment and activation of testicular macrophages, thereby causing an inflammatory response and promoting apoptosis, ultimately leading to decreased semen quality and conception. The relative expression of inflammatory factors was reduced to varying degrees by Huaji Jianpi Decoction. It was hypothesized that Huaji Jianpi Decoction may improve male semen quality by inhibiting the inflammatory response in vivo and reducing excessive apoptosis of germ cells.
In conclusion, we observed that Huaji Jianpi Decoction could inhibited TNF-α and MCP-1 mRNA expression caused by obesity and suppressed obesity-induced hypogonadism in mice via reducing TNF-α and MCP-1 mRNA expression. Our results suggest that Huaji Jianpi Decoction can improve the reproductive function of obese mice by improving the level of lipid metabolism. Our study may provide a new theoretical basis for the future clinical treatment of reproductive system diseases in obese males.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Funding: This work was supported by the Natural Science Foundation of Hebei Province (No. H208201218); Science and Technology Research Key Project of Higher Education Department of Hebei Province (No. ZD2020101); NHC Key Laboratory of Family Planning and Healthy, Hebei Key Laboratory of Reproductive Medicine, Hebei Research Institute for Family Planning Science and Technology Open Project (No. SZ-202003); and Medical Discipline Cultivation Project of Hebei University (No. 2020B04).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-115/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-115/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-115/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal research was approved by the Ethical Committee of Hebei University (approval ID: IACUC-2021XS038). The animal research was performed in compliance with the Hebei University Laboratory Animal Welfare and Ethics guidelines of the Animal Welfare and Ethical Committee of Hebei University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vandevijvere S, Jaacks LM, Monteiro CA, et al. Global trends in ultraprocessed food and drink product sales and their association with adult body mass index trajectories. Obes Rev 2019;20:10-9. [Crossref] [PubMed]
- Malcher A, Rozwadowska N, Stokowy T, et al. The gene expression analysis of paracrine/autocrine factors in patients with spermatogenetic failure compared with normal spermatogenesis. Am J Reprod Immunol 2013;70:522-8. [Crossref] [PubMed]
- Spradley FT, Wilson BA, Martin HL, et al. Impact of chronic hyperleptinemia on placental ischemia-induced hypertension in pregnant rats. FASEB J 2020;34:S1. [Crossref]
- Abdel-Fadeil MR, Abd Allah ESH, Iraqy HM, et al. Experimental obesity and diabetes reduce male fertility: Potential involvement of hypothalamic Kiss-1, pituitary nitric oxide, serum vaspin and visfatin. Pathophysiology 2019;26:181-9. [Crossref] [PubMed]
- Zhang R, Zhu X, Bai H, et al. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front Pharmacol 2019;10:123. [Crossref] [PubMed]
- Andreotti F, Crea F, Hennekens CH. Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med 2017;376:1490-1. [Crossref] [PubMed]
- Leisegang K, Sengupta P, Agarwal A, et al. Obesity and male infertility: Mechanisms and management. Andrologia 2021;53:e13617. [Crossref] [PubMed]
- Li J, Wang Y, Bian C, et al. Vertical banding gastric volume reduction to improve the fertility of obese male infertile rats. Research in Surgery & New Technology 2018;7:89-92.
- Rowland DL, McNabney SM, Mann AR. Sexual function, obesity, and weight loss in men and women. Sex Med Rev 2017;5:323-38. [Crossref] [PubMed]
- Rastrelli G, Lotti F, Reisman Y, et al. Metabolically healthy and unhealthy obesity in erectile dysfunction and male infertility. Expert Rev Endocrinol Metab 2019;14:321-34. [Crossref] [PubMed]
- Hammoud AO, Meikle AW, Reis LO, et al. Obesity and male infertility: a practical approach. Semin Reprod Med 2012;30:486-95. [Crossref] [PubMed]
- Oliveira PF, Sousa M, Silva BM, et al. Obesity, energy balance and spermatogenesis. Reproduction 2017;153:R173-85. [Crossref] [PubMed]
- Salas-Huetos A, James ER, Broberg DS, et al. The combined effect of obesity and aging on human sperm DNA methylation signatures: inclusion of BMI in the paternal germ line age prediction model. Sci Rep 2020;10:15409. [Crossref] [PubMed]
- Zhang J, Yang B, Cai Z, et al. The Negative Impact of Higher Body Mass Index on Sperm Quality and Erectile Function: A Cross-Sectional Study Among Chinese Males of Infertile Couples. Am J Mens Health 2019;13:1557988318822572. [Crossref] [PubMed]
- Wang H, Zhao R, Guo C, et al. Knockout of BRD7 results in impaired spermatogenesis and male infertility. Sci Rep 2016;6:21776. [Crossref] [PubMed]
- Ilacqua A, Izzo G, Emerenziani GP, et al. Lifestyle and fertility: the influence of stress and quality of life on male fertility. Reprod Biol Endocrinol 2018;16:115. [Crossref] [PubMed]
- Darbandi M, Darbandi S, Agarwal A, et al. Reactive oxygen species and male reproductive hormones. Reprod Biol Endocrinol 2018;16:87. [Crossref] [PubMed]
- Chen Y, Jiao N, Jiang M, et al. Loganin alleviates testicular damage and germ cell apoptosis induced by AGEs upon diabetes mellitus by suppressing the RAGE/p38MAPK/NF-κB pathway. J Cell Mol Med 2020;24:6083-95. [Crossref] [PubMed]
- Chang HH, Eibl G. Obesity-Induced Adipose Tissue Inflammation as a Strong Promotional Factor for Pancreatic Ductal Adenocarcinoma. Cells 2019;8:673. [Crossref] [PubMed]
- Al-Rashed F, Ahmad Z, Iskandar MA, et al. TNF-α Induces a Pro-Inflammatory Phenotypic Shift in Monocytes through ACSL1: Relevance to Metabolic Inflammation. Cell Physiol Biochem 2019;52:397-407. [Crossref] [PubMed]
- Sharma S, Hanukoglu I. Mapping the sites of localization of epithelial sodium channel (ENaC) and CFTR in segments of the mammalian epididymis. J Mol Histol 2019;50:141-54. [Crossref] [PubMed]
- Xu YR, Dong HS, Yang WX. Regulators in the apoptotic pathway during spermatogenesis: Killers or guards? Gene 2016;582:97-111. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


