
The efficacy and safety of dutasteride and finasteride in patients with benign prostatic hyperplasia: a systematic review and meta-analysis
Introduction
Benign prostatic hyperplasia (BPH) is a common disease in middle-aged and elderly men. A meta-analysis has shown that the incidence of BPH in Chinese men increases significantly with age, with an incidence of 69.2% in men over 80 years old (1). There are many treatments for BPH: (I) drug therapy, including alpha blockers, 5-alpha reductase inhibitors, and combination drug therapy; (II) minimally invasive therapies, such as transurethral microwave thermotherapy, transurethral needle ablation, homium laser enulcleation of prostate; (III) surgery procedures include: transurethral resection of the prostate, transurethral incision of the prostate, simple prostatectomy, laser surgery. Drug therapy is one of the curative treatments for BPH. Finasteride and dutasteride are the most frequently considered in treating BPH. Finasteride is a 5α-reductase (5α-R) inhibitor, which is the first-line therapy for BPH. Nevertheless, it has been reported that finasteride can increase the risk of loss of libido and ejaculatory dysfunction (2,3). Dutasteride is a 5α-R inhibitor as well. It has been found that dutasteride has advantages in improving symptoms related to prostatic hyperplasia and reducing acute urinary retention in the treatment of BPH (4,5). The 5α-R inhibitor decreases the level of dihydrotestosterone (DHT), which is responsible for prostate growth. Finasteride reduces 70% of circulating DHT levels, while dutasteride almost completely reduces DHT levels in both the serum and the prostate. A study found that in treating BPH, compared with finasteride, dutasteride showed a greater decrease in prostate-specific antigen (PSA) and International Prostate Symptom Score (IPSS) (6). Whereas, results of Yin et al. suggested no significant differences between dutasteride and finasteride in treating BPH, except dutasteride improves BPH symptoms in IPSS (7). Therefore, this study systematically compared the efficacy of dutasteride and finasteride for BPH to provide medical evidence for clinical treatment.
We present the following article in accordance with the PRISMA reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-58/rc).
Methods
Inclusion criteria
Patients
Men between the ages of 50 and 70 with obvious symptoms of prostatic hyperplasia [IPSS >8; average urination time <12 mL/s; a diagnosis of prostate hyperplasia confirmed by prostate B-ultrasound or computed tomography (CT) examination] were identified as subjects (8).
Intervention
Intervention was based on dutasteride or finasteride were included in our study.
Comparator
Comparator was the pharmacological therapy that either dutasteride or finasteride applied to patients.
Outcomes
Outcomes included the assessment of IPSS, maximum urinary flow rate (Qmax), prostate volume (PV), quality of life (QOL), PSA and adverse drug reactions (ADRs).
Study design
Study design was randomized controlled trials (RCTs) of dutasteride versus finasteride in the treatment of BPH. The origin of the scientific legend was traced, and the language was limited to English or Chinese.
Exclusion criteria
(I) Reviews, case-control studies, systematic evaluations and letters were excluded; (II) duplicate publications or articles with no available data were excluded.
Search strategy
A search of relevant articles was conducted from January 2009 to July 2021 using the electronic databases PubMed, Embase, Medline, Cochrane Library, China Academic Journals Full-text Database (CJFD), Chinese Science and Technology Journal Database (VIP) and Wanfang Database. Search terms included “Benign Prostatic hyperplasia”, “Random”, “Control”, “Dutasteride”, “Finasteride”, “Adult” and “Male”.
Data extraction and quality assessment
Two researchers independently screened the titles and abstracts for eligibility. When there was a difference of opinion, a third reviewer was consulted. The authors were contacted about missing or unclear data. The risk of bias and literature quality were evaluated according to the Cochrane Systematic Review (9): (I) RCT; (II) allocation scheme; (III) blind method; (IV) complete data; (V) selection bias; and (VI) other biases. For RCTs, we used the Jadad scale with the classification criteria of high quality (3 or more) and low quality (2 or less).
Statistical analysis
Meta-analyses were carried out using RevMan version 5.0 statistical processing software. The presence of substantial heterogeneity was assessed. If the P value was >0.1, the test of homogeneity was statistically significant, and then the fixed effects model was adopted. On the other hand, the random effects model was adopted if there was heterogeneity. Mean difference (MD) or relative risk (RR) and 95% confidence intervals (CIs) were used to analyze the end indices. A two-sided P value <0.05 was considered to indicate statistical significance.
Results
Characteristic of eligible studies
A total of 240 potentially relevant articles were selected, including 28 Chinese articles and 212 English articles. After reading the abstracts and titles, 222 publications were excluded. Of the remaining 18 studies, 10 were excluded due to being non-RCTs or having incomplete data or an absence of BPH disease Finally, 8 RCTs comparing the efficacy of dutasteride and finasteride in the treatment of BPH over 6 months of treatment or longer were included in this meta-analysis; 2,116 subjects were involved. The study selection process is illustrated in Figure 1. The main characteristics of the 8 studies are presented in Table 1.
Table 1
| First author (year) | Study design | Experimental group | Control group | Cases | Age, years | Intervention | Course, months | Outcome indicator | Jadad score |
|---|---|---|---|---|---|---|---|---|---|
| Kuang CQ (10), 2015 | RCT | Dutasteride | Finasteride | Dutasteride (n=28) | >60 | Dutasteride, 0.5 mg, qd | 6 | PV, Qmax, IPSS, QOL, ADR | 4 |
| Finasteride (n=28) | Finasteride, 5 mg, qd | ||||||||
| Peng T (11), 2015 | RCT | Dutasteride | Finasteride | Dutasteride (n=39) | ≥60 | Dutasteride, 0.5 mg, qd | >6 | PV, IPSS, QOL, Qmax | 3 |
| Finasteride (n=45) | Finasteride, 5 mg, qd | ||||||||
| Li YZ (12), 2013 | RCT | Dutasteride | Finasteride | Dutasteride (n=36) | ≥60 | Dutasteride, 0.5 mg, qd | 6 | PV, IPSS, QOL, Qmax, PSA, ADR | 4 |
| Finasteride (n=36) | Finasteride, 5 mg, qd | ||||||||
| Nickel JC (13), 2011 | RCT | Dutasteride | Finasteride | Dutasteride (n=813) | >50 | Dutasteride, 0.5 mg, qd | 12 | PV, Qmax, ADR | 3 |
| Finasteride (n=817) | Finasteride, 5 mg, qd | ||||||||
| Sciarra A (14), 2010 | RCT | Dutasteride | Finasteride | Dutasteride (n=20) | >50 | Dutasteride, 0.5 mg, qd | 6 | PSA | 4 |
| Finasteride (n=20) | Finasteride, 5 mg, qd | ||||||||
| Clark RV (15), 2004 | RCT | Dutasteride | Finasteride | Dutasteride (n=57) | >50 | Dutasteride, 0.5 mg, qd | 6 | ADR | 4 |
| Finasteride (n=55) | Finasteride, 5 mg, qd | ||||||||
| Jeong YB (16), 2009 | RCT | Dutasteride | Finasteride | Dutasteride (n=40) | >50 | Dutasteride, 0.5 mg, qd | 12 | PV, IPSS, PSA | 3 |
| Finasteride (n=37) | Finasteride, 5 mg, qd | ||||||||
| Qian X (17), 2015 | RCT | Dutasteride | Finasteride | Dutasteride (n=16) | ≥60 | Dutasteride, 0.5 mg, qd | 36 | PV, Qmax, IPSS, QOL, PSA | 4 |
| Finasteride (n=29) | Finasteride, 5 mg, qd |
RCT, randomized controlled trial; qd, once a day; PV, prostate volume; Qmax, maximum urinary flow rate; IPSS, International Prostate Symptom Score; QOL, quality of life; PSA, prostate-specific antigen; ADR, adverse drug reaction.
Quality assessment and risk of bias assessment
The Jadad scores of the 8 included articles were all greater than 3, as shown in Table 1. The risks of bias of the 8 included studies are shown in Figures 2,3.

In the Cochrane risk of bias (RoB 2.0) analysis, all literature had no data miss and selection bias. Most literature was a low risk of bias. Little literature had high-risk selection bias and performance bias (Figure 2).
Only one literature had a high-risk bias in selection bias and another one literature had performance bias individually (Figure 3).
Meta-analysis results of outcomes
IPSS
Five RCTs (10-12,16,17) were included to analyze the IPSS scores after treatment. There was no significant heterogeneity (P=0.63; I2=0%). The forest plots indicated that there was no significant difference in IPSS between the subset analyses of BPH patients who were administered dutasteride versus finasteride [MD =0.13; 95% CI: (−0.55, 0.82); P=0.70] (Figure 4).

Qmax
Five RCTs (10-13,16) with a total of 1,887 patients were included to analyze Qmax after treatment. There was no significant heterogeneity (P=0.57; I2=0%). The forest plots indicated a significantly greater increase in Qmax in the dutasteride group than in the finasteride group [MD =0.32; 95% CI: (0.01, 0.63); P=0.04] (Figure 5).
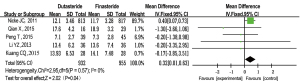
PV
Six RCTs (10-15) with a total of 1,964 patients were included to analyze PV after treatment. There was no significant heterogeneity (P=0.84; I2=0%). The forest plots indicated that there was no significant difference in PV between the subset analyses of BPH patients who were administered dutasteride versus finasteride [MD =−1.25; 95% CI: (−3.30, 0.79); P=0.23] (Figure 6).

QOL
Four RCTs (10-12,17) were included to analyze the QOL of these patients after treatment. There was significant heterogeneity (P=0.01; I2=73%). The random effects model was used. The forest plots indicated that there was no significant difference in QOL between the subset analyses of BPH patients who were administered dutasteride versus finasteride [MD =−0.44; 95% CI: (−0.93, 0.05); P=0.08] (Figure 7).
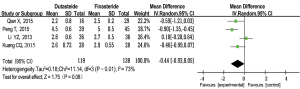
Serum PSA level
Four RCTs (12,13,16,17) were included to analyze the serum PSA levels after treatment. There was no significant heterogeneity (P=0.70; I2=0%). The forest plots indicated that there was no significant difference in the serum PSA levels between the subset analyses of BPH patients who were administered dutasteride versus finasteride [MD =−0.04; 95% CI: (−0.15, 0.07); P=0.50] (Figure 8).

ADRs
Four RCTs (10-13,15) with a total of 1,870 patients were included to analyze adverse reactions. There was no significant difference in heterogeneity (P=0.73; I2=0%). The forest plots indicated that there was no significant difference in ADRs between the subset analyses of BPH patients who were administered dutasteride versus finasteride [MD =−0.01; 95% CI: (−0.05, 0.04); P=0.72] (Figure 9).

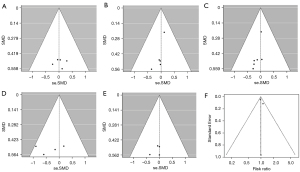
Publication bias
Funnel plots were drawn based on the literature whose main indicators are IPSS, Qmax, PV, QOL, serum PSA, and adverse events indicating that there was no significant publication bias and that the results were mostly stable and reliable (Figure 10). However, considering that few studies were included in this meta-analysis and most of them dispersed at the bottom of funnel plots, publication bias cannot be completely ruled out.
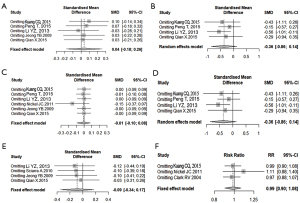
Sensitivity analysis
As indicated in Figure 11, the sensitivity analysis of the meta-analysis literature that included IPSS, Qmax, PV, QOL, serum PSA, and adverse events individually, the significance of the combined effect sizes did not change significantly after the corresponding literature for each indicator was excluded from inclusion in turn. That means there were no extremes in the included studies.
Discussion
BPH is a common disease in men over 50 years old, and its incidence increases with age (18). The main clinical manifestations of BPH are lower urinary tract symptoms, enlarged PV, decreased peak urine flow, high IPSS score, and increased serum PSA (19). DHT whose formation is catalyzed by the enzyme 5α-R, plays a vital role in the progression of BPH (20,21). 5α-R inhibitors can effectively reduce the concentration of DHT in the prostate and promote prostate smooth muscle contraction (22-24). As 5α-R inhibitors, dutasteride and finasteride are mainly used to improve the symptoms of prostatic hyperplasia. 5α-R is a protease that can convert testosterone to DHT and accelerate the progression of prostate hyperplasia. It has two isoenzymes. Dutasteride is a selective inhibitor of both type I and type II isoenzymes of 5α-R, whereas finasteride selectively inhibits the type II isoform (25). Although the efficacy of monotherapy has been established clinically (26,27), the efficacy of the two drugs has not been compared. Therefore, this study systematically examined and compared the efficacy of the two drugs in the treatment of BPH with a meta-analysis.
Eight RCTs involving 2,116 participants were included to compare the efficacy of dutasteride (0.5 mg/day) versus finasteride (5 mg/day) in the treatment of BPH over a period of 6 months. The meta-analysis showed that compared with finasteride, dutasteride could effectively improve Qmax in patients with BPH [MD =0.32; 95% CI: (0.01, 0.63); P=0.04], and the difference was statistically significant. IPSS [MD =0.13; 95% CI: (−0.55, 0.82); P=0.70], PV [MD =−1.25; 95% CI: (−3.30, 0.79); P=0.23), QOL [MD =−0.44; 95% CI: (−0.93, 0.05); P=0.08], serum PSA level [MD =−0.04; 95% CI: (−0.15, 0.07); P=0.5] and the occurrence of ADRs [RR =−0.01; 95% CI: (−0.05, 0.04); P=0.72] showed no significant difference between the two groups. Thus, dutasteride is more effective than finasteride for improving the maximum urine flow rate in patients with BPH. No significant difference was found between dutasteride and finasteride in improving symptoms, PV, reducing PSA level and QOL, or the occurrence of ADRs. Dutasteride is an effective treatment for BPH. Qmax represents the maximum urine flow rate of patients with prostatic hyperplasia, indicating the degree of prostatic hyperplasia, such as BPH (28,29). Our results showed that dutasteride effectively improved Qmax in patients with BPH compared with finasteride [MD =0.32; 95% CI: (0.01, 0.63); P=0.04]. This suggests that dutasteride may be superior to finasteride in improving Qmax in BPH patients in clinical. BPH is also characterized by increased IPSS, PV, QOL and serum PSA levels. High levels of serum PSA promote the progression of prostatic hyperplasia. Previous results showed that, compared with placebo, both dutasteride and finasteride reduced IPSS and QOL and increased PV and serum PSA levels (30,31). There was no significant difference in ADRs between the dutasteride and finasteride groups in our analysis. However, it has been reported that long-term treatment with dutasteride leads to erectile dysfunction, decreased testosterone levels, increased glucose and glycosylated hemoglobin, and changes in the blood lipid profile, suggesting metabolic imbalance and decreased gonadal function (32). Therefore, it is advisable to explain the potential serious side effects of long-term dutasteride therapy to patients prior to initiation of dutasteride therapy.
To a certain degree, there are some limitations and shortcomings in this study. First, there are differences in patient selection and experimental design among studies, resulting in greater heterogeneity in some indicators. Second, the follow-up term of each study was different. Some studies even had no long-term follow-up data. Thus, the long-term efficacy cannot be analyzed. Finally, the articles included were mainly English, which may affect selection bias.
In conclusion, dutasteride is an effective and safe treatment for BPH, with a better effect on improving Qmax than finasteride. Due to the limitations of the methodological quality and sample size of the included studies, this conclusion needs to be verified by stratified RCTs with high volumes and long follow-up times.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-58/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-58/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang W, Guo Y, Zhang D, et al. The prevalence of benign prostatic hyperplasia in mainland China: evidence from epidemiological surveys. Sci Rep 2015;5:13546. [Crossref] [PubMed]
- Tacklind J, Fink HA, Macdonald R, et al. Finasteride for benign prostatic hyperplasia. Cochrane Database Syst Rev 2010;CD006015. [PubMed]
- Zhou Z, Cui Y, Wu J, et al. Meta-analysis of the efficacy and safety of combination of tamsulosin plus dutasteride compared with tamsulosin monotherapy in treating benign prostatic hyperplasia. BMC Urol 2019;19:17. [Crossref] [PubMed]
- Ciríaco SL, Carvalho IPS, Alves Terceiro Neto J, et al. Development of microemulsion of tamsulosin and dutasteride for benign prostatic hyperplasia therapy. Colloids Surf B Biointerfaces 2020;185:110573. [Crossref] [PubMed]
- Kosilov KV, Kuzina IG, Kuznetsov V, et al. Improvement of the symptoms of lower urinary tract and sexual dysfunction with tadalafil and solifenacin after the treatment of benign prostatic hyperplasia with dutasteride. Prostate Int 2020;8:78-84. [Crossref] [PubMed]
- Zhou Z, Cui Y, Wu J, et al. Efficacy and safety of dutasteride compared with finasteride in treating males with benign prostatic hyperplasia: A meta-analysis of randomized controlled trials. Exp Ther Med 2020;20:1566-74. [Crossref] [PubMed]
- Yin T, Qiao Z, Li Y, et al. Comparisons of the Efficacy and Safety of Finasteride and Dutasteride for Benign Prostatic Hyperplasia: A Network Meta-Analysis. Am J Ther 2017;24:e517-23. [Crossref] [PubMed]
- Langan RC. Benign Prostatic Hyperplasia. Prim Care 2019;46:223-32. [Crossref] [PubMed]
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions Version 5.1.0. The Cochrane collaboration, 2011. Naunyn Schmiedebergs Arch Fur Exper 2011;2:126-30.
- Kuang CQ, Guo L, Chen C, et al. Evaluation of the clinical efficacy of dutasteride in the treatment of benign prostatic hyperplasia. Journal of North Pharmacy 2015;12:59.
- Peng T, Zhu LJ, Shao HB. Application of finasteride and dutasteride in transurethral plasmakinetic prostatectomy. Anhui Medical Journal 2015;36:1507-9.
- Li YZ, Wang J. Clinical efficacy and safety analysis of Dutasteride in treatment of benign prostatic hyperplasia. Chinese Journal of Andrology 2013;(7):49-51, 55.
- Nickel JC, Gilling P, Tammela TL, et al. Comparison of dutasteride and finasteride for treating benign prostatic hyperplasia: the Enlarged Prostate International Comparator Study (EPICS). BJU Int 2011;108:388-94. [Crossref] [PubMed]
- Sciarra A, Salciccia S, Nesi G, et al. Comparative effect of finasteride and dutasteride on chromogranin A levels. Anticancer Res 2010;30:4737-42. [PubMed]
- Clark RV, Hermann DJ, Cunningham GR, et al. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab 2004;89:2179-84. [Crossref] [PubMed]
- Jeong YB, Kwon KS, Kim SD, et al. Effect of discontinuation of 5alpha-reductase inhibitors on prostate volume and symptoms in men with BPH: a prospective study. Urology 2009;73:802-6. [Crossref] [PubMed]
- Qian X, Yu G, Qian Y, et al. Efficacy of 5α-reductase inhibitors for patients with large benign prostatic hyperplasia (>80 mL) after transurethral resection of the prostate. Aging Male 2015;18:238-43. [Crossref] [PubMed]
- Challacombe B, Sabharwal T. Prostate artery embolisation for benign prostatic hyperplasia. BMJ 2018;361:k2537. [Crossref] [PubMed]
- Kuzmenko AV, Kuzmenko VV, Gyaurgiev TA. Combination drug therapy in patients with BPH. Urologiia 2018;101-5. [Crossref] [PubMed]
- Madersbacher S, Sampson N, Culig Z. Pathophysiology of Benign Prostatic Hyperplasia and Benign Prostatic Enlargement: A Mini-Review. Gerontology 2019;65:458-64. [Crossref] [PubMed]
- Pejčić T, Tosti T, Tešić Ž, et al. Testosterone and dihydrotestosterone levels in the transition zone correlate with prostate volume. Prostate 2017;77:1082-92. [Crossref] [PubMed]
- Zhang H, Frendl DM, Wang Z, et al. High Real-World Medication Adherence and Durable Clinical Benefit in Medicare Patients Treated with 5-Alpha Reductase Inhibitors for Benign Prostatic Hyperplasia. J Urol 2020;204:325-31. [Crossref] [PubMed]
- Wallner LP, DiBello JR, Li BH, et al. The Use of 5-Alpha Reductase Inhibitors to Manage Benign Prostatic Hyperplasia and the Risk of All-cause Mortality. Urology 2018;119:70-8. [Crossref] [PubMed]
- Cardarelli-Leite L, de Assis AM, Moreira AM, et al. Impact of 5-Alpha-Reductase Inhibitors Use at the Time of Prostatic Artery Embolization for Treatment of Benign Prostatic Obstruction. J Vasc Interv Radiol 2019;30:228-32. [Crossref] [PubMed]
- Ravish IR, Nerli RB, Amarkhed SS. Finasteride to evaluate the efficacy of dutasteride in the management of patients with lower urinary tract symptoms and enlarged prostate. Arch Androl 2007;53:17-20. [Crossref] [PubMed]
- Busetto GM, Del Giudice F, D'Agostino D, et al. Efficacy and safety of Finasteride (5 alpha-reductase inhibitor) monotherapy in patients with benign prostatic hyperplasia: A critical review of the literature. Arch Ital Urol Androl 2020;91:205-10. [Crossref] [PubMed]
- Eisen C, Lulic Z, Palacios-Moreno JM, et al. Persistence and adherence to dutasteride/tamsulosin fixed-dose versus free-combination alpha blocker/5ARI therapy in patients with benign prostate hyperplasia in Germany . Int J Clin Pharmacol Ther 2020;58:37-49. [Crossref] [PubMed]
- Singh I, Tk A, Gupta S. Efficacy and safety of tadalafil vs tamsulosin in lower urinary tract symptoms (LUTS) as a result of benign prostate hyperplasia (BPH)-open label randomised controlled study. Int J Clin Pract 2020;74:e13530. [Crossref] [PubMed]
- O'Leary MP, Roehrborn C, Andriole G, et al. Improvements in benign prostatic hyperplasia-specific quality of life with dutasteride, the novel dual 5alpha-reductase inhibitor. BJU Int 2003;92:262-6. [Crossref] [PubMed]
- Haque N, Masumori N, Sakamoto S, et al. Superiority of dutasteride 0.5 mg and tamsulosin 0.2 mg for the treatment of moderate-to-severe benign prostatic hyperplasia in Asian men. Int J Urol 2018;25:944-51. [Crossref] [PubMed]
- Ghadian A, Rezaei M. Combination therapy with omega-3 fatty acids plus tamsulocin and finasteride in the treatment of men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH). Inflammopharmacology 2017;25:451-8. [Crossref] [PubMed]
- Traish A, Haider KS, Doros G, et al. Long-term dutasteride therapy in men with benign prostatic hyperplasia alters glucose and lipid profiles and increases severity of erectile dysfunction. Horm Mol Biol Clin Investig 2017;30. j/hmbci. [Crossref] [PubMed]




