
Design of a fully intraureteral stent and proof-of-concept in vivo evaluation
Introduction
Ureteral stents are employed frequently to facilitate urine drainage disrupted by obstructions due to ureteral stones and either intrinsic or extrinsic ureteral obstruction. They are also used following other endourological and surgical procedures on the urinary tract, to assist urine flow until edema decreases and incisions heal (1).
Ureteral stents were employed regularly via cystoscopic insertion (2) from about 1967, but routine use began in 1978 with the introduction of the double-J (pigtail) stent by Finney and by Hepperlen et al. (3,4). Since then, studies have noted the significant, negative effects of ureteral stents on patient quality of life, with stent-related symptoms affecting daily activities of more than 80% of patients (5). Indeed, poor toleration of double-J ureteral stents, with symptoms involving urinary frequency, urgency, dysuria, incontinence, hematuria, incomplete emptying, a feeling of pelvic heaviness, and lumbar pain is well-documented (5-9). These symptoms are generally believed to be related to anchoring stent ends, which are usually curled, located in the bladder and in the kidney.
Many improvements to ureteral stent design and composition have been introduced since 1978, particularly in terms of the physical and chemical properties of the material. Novel biodegradable and coated ureteral stent designs have received special attention (1,10). These stents aim to reduce inflammatory processes and microbial adherence to the stent, to minimize tissue irritation. And yet, notwithstanding the recognition that kidney and bladder anchors may be the major cause of patient discomfort, it should be noted that the fundamental double-J design has remained essentially unchanged. Recent efforts to address this aspect have focused on removing the distal J anchor (11-13) or otherwise minimizing stent material in the bladder (14-16).
The purpose of this study was to examine the potential feasibility and safety of a newly-designed ureteral stent that resides completely within the ureter. An initial animal study allowed testing of stent function and safety, in particular to ensure straightforward endoscopic insertion and free urine drainage, and to examine possible undesirable symptoms that include stent migration, urinary tract inflammation and/or hydronephrosis, or overt stress related changes in animal behavior in terms of activity, mobility, appetite, and sleep patterns. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-41/rc).
Methods
Ureteral stent design and preparation
An innovative stent design is proposed, aiming to offer the same functionality and ease of insertion/removal as conventional double-J stents, but with additional features. The stent, including the two in-plane spiral curls, named “YotiCurl”, is designed to reside entirely within the ureter, distal to the renal pelvis and proximal to the bladder (Figure 1). A standard suture is attached to the distal spiral (not shown in the picture), similar to that used in commercially-available double-J stents, that extends into the bladder or through the urethra, to facilitate stent removal. Prototype stents with the new design were synthesized readily by modifying commercially available, radiopaque, 6F copolymeric, biocompatible ureteral stents, with hydrophilic coating. The length of the stent can vary, i.e., from ~5 to ~15 cm including the curls, to allow guidewire placement of the spiral curls proximal and distal to an obstruction, but entirely within the ureter, virtually regardless of obstruction location along the ureter.
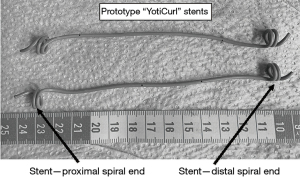
The spiral curl design is intended to maintain the integrity of the ureteral lumen and enable free urine flow in the ureter lumen enclosing the spiral, while also permitting the stent lumen itself to facilitate urine flow, similar to the action of conventional double-J stents. Initial in vitro testing of prototype stent designs, via injection of a solution containing dye tracer directly into the proximal opening of the YotiCurl stent, and then into a tube mimicking a ureter that enclosed the stent, indicated that fluid flows freely within the stent itself as well as through the simulated ureter. More specifically, 30 cm length of clear, flexible silicone tube (7 mm inner diameter) was placed in a deep flat tray filled with water. A 0.035” Sensor guidewire (Boston Scientific, Marlborough, MA, USA) was inserted into a ~15 cm long YotiCurl stent, and then into the silicone tube. The stent was placed within the tubing such that both proximal and distal ends lay within the tube; the guidewire was then slowly withdrawn and opening of the two spiral curls, within the confines of the tube, was confirmed visually. Green food coloring in water was first injected into the YotiCurl stent, directly through the proximal opening, by connecting it directly to a syringe via a narrow tube. Dye was seen to exit through several side holes along the length of the stent, and through the distal curl, indicating free fluid flow through the stent lumen. Dye was subsequently injected directly into the proximal inlet of the tube, mimicking the ureter lumen, and free fluid flow to the distal tube outlet was observed.
Methods and experiment
Experiments were performed under a project license (No. 29-2021) granted by the Shamir Medical Center Institutional Animal Care and Use Committee (IACUC), in compliance with institutional guidelines for the care and use of animals. A first test designed to confirm the intended expansion of the spiral curls in a ureter was performed on a pig cadaver. A proof-of-concept in vivo evaluation in a single pig model was then completed, with stent insertions into both ureters, to test stent viability.
The preliminary experiment involved insertion of a 7 cm long YotiCurl stent into a ureter of a pig cadaver (GLPigs facility, Pre-Clinical R&D Department). The pig (female domestic pig, Sus scrofa domestica) was the subject of a separate procedure, unrelated to the urinary tract; it had been euthanized shortly before the stent test, followed by laparotomy to examine other abdominal organs. In this first YotiCurl test, the urinary tract was exposed gently but not disrupted; the prototype stent was then placed by direct guidewire insertion to the ureter, with simultaneous visual observation of stent placement and spiral curl expansion.
In vivo stent insertion and emplacement tests in two ureters as shown in Figure 1 were subsequently performed on a live pig, over a 25-day period (July 2021). The experiment was carried out in the GLPigs facility, Pre-Clinical R&D Department. A female domestic pig (Sus scrofa domestica, weight 54 kg) was admitted 6 days earlier for acclimation.
Prior to use in the live animal model, the synthesized stents were disinfected in CIDEX® solution; this treatment was considered suitable given the expected overall low risk of infection. For future clinical testing, the stents can be sterilized by standard means used by commercial manufacturers.
The experiment proceeded as follows. General anesthesia consisted of premedication with 20 mg/kg intramuscular Ketamine HCl (Bremer pharma GMBH, Warburg, Germany) and 2 mg/kg intramuscular Xylazine HCl (Eurovet Animal Health, B.V. Bladel, The Netherlands), followed by 7.5 mg/pig intravenous Midazolam (Rafa Laboratories, Jerusalem, Israel); induction was isoflurane (Piramal Critical Care, Inc., Bethlehem, PA, USA) via mask, with maintenance by tracheal intubation with 1–2% isoflurane, and intermittent positive pressure ventilation using 100% O2. The same anesthesia was applied for each of the interventional procedures and follow-up checks.
With the pig lying in the supine position, a 22F rigid cystoscope (Karl Storz SE & Co. KG, Tuttlingen, Germany) was advanced transurethrally. The ureteral orifices were identified and a 0.035” Sensor guidewire (Boston Scientific) was advanced through the right ureter up to the renal collecting system, under fluoroscopy and pyelography using 5 mL of contrast material (Omnipague/Iohexol, GE Healthcare, Dublin, Ireland). One YotiCurl stent was inserted (Day 1, weight 58 kg) to the right ureter, approximately centered between the ureteropelvic and ureterovesical junctions, using standard endourological procedures with a radiopaque stent and pusher. Under intravenous contrast pyelography with 20 mL Omnipague/Iohexol (GE Healthcare), stent placement and opening of the spiral curl ends within the ureter was confirmed. In parallel, from Day 1, 150 mg/pig Marbofloxacin, (Vetoquinol, Chêne Sainte-Anne, France) was administered intravenously, once a day, for 8 days.
Two days later (Day 3), via intravenous pyelography, comparing to the Day 1 images, the positioning, geometry of the spirals, and lack of migration of the first stent, as well as free urine drainage and lack of hydronephrosis were confirmed. In particular, urine drainage was confirmed by use of contrast agent injected intravenously. Within a few minutes, the contrast agent was observed simultaneously in both collecting systems, with accumulation in the bladder, indicating no evidence of obstruction. This early-stage observation, validating apparent animal well-being, enabled continuation of the experiment according to the approved experiment protocol. A second YotiCurl stent was then inserted into the second (left) ureter, somewhat more proximal in the ureter than the right stent, to explore stent behavior in a different location within the ureter. Stent positioning was again confirmed by radiography (Figure 2).
Twice-daily checks of animal well-being by the veterinary staff to observe activity, appetite, and sleep patterns, and at least weekly checks of the animal by a veterinarian, were recorded. In parallel, four weekly follow-ups (Days 5, 11, 18, 25) were performed, all involving intravenous pyelography, to evaluate stent location, spiral curl integrity, urine drainage in both ureters, ureter dilatation, and possible hydronephrosis, the latter two by comparison to radiographs from Day 1. Drainage was again based on observing contrast excretion from the kidneys and accumulating in the bladder, time of clearance, and lack of hydronephrosis. More specifically, on each weekly follow-up (Day 5 and thereafter), with both YotiCurl stents in place, contrast agent was again injected and observed in the collecting systems and the bladder within minutes. These results indicated that no obstruction nor delay was apparent in the presence of both YotiCurl stents. At completion of the last check (Day 25, weight 66.2 kg), explorative laparotomy was done for inspection and the entire urinary tract was examined visually. In a deeply anesthetized animal, a lethal dose of 20 mL potassium chloride 14.9% (B. Braun Melsungen AG, Melsungen, Germany) was then injected intravenously. After euthanasia, the kidneys, ureters and bladder section were harvested, placed in 4% formaldehyde, and prepared for analysis by a pathologist.
A pathologist analyzed histology of 13 samples from the entire urinary tract. In particular, tissue samples were taken from the renal pelvis of each kidney, and from the bladder in the region close to both ureteral orifices. In each of the two ureters, samples that included both ureter and stent were sectioned in five locations: proximal to the proximal spiral, at the site of the proximal spiral, along the mid-ureteral region exposed to the straight portion of the stent, at the site of the distal spiral, distal to the distal spiral. The pathologist evaluated, visually from standard sample and slide preparation, standard histological measures that included examination of all urinary tract tissue layers and presence of inflammatory cells (eosinophils and lymphocytes).
Finally, proximal and distal spiral curls, and straight sections of the stents present in the sectioned ureter-stent samples, which were not required for additional histology, were examined visually for possible encrustation.
Results
The YotiCurl stent was inserted smoothly and easily into a ureter of the pig cadaver, and the proximal and distal spiral curl ends opened naturally and without perforating or otherwise causing negative effects to the ureter. Moreover, ease of stent removal was confirmed, with the spiral curls releasing and straightening in a manner similar to conventional pigtails when pulled from the distal end.
Similarly, the initial insertion and emplacement of both stents in the in vivo experiment was smooth and uneventful. Based on this experience, to achieve consistent positioning of the YotiCurl suggest, it is suggested that the urinary system first be delineated by either retrograde or antegrade pyelogram using contrast agent. Then, under fluoroscopy, the proximal tip of the YotiCurl should be located ~3 cm above the level of obstruction—either a stone or a stricture—before the sensor wire is withdrawn. This procedure will allow the spiral curls of the stent to be situated above and beneath the obstruction.
During the course of the experiment, based on regular radiography analyses using contrast injection (Figure 2), and comparing radiographic images over the duration of the experiment, both stents remained in their initial location, with essentially no migration, and little to no hydronephrosis was noted. Moreover, the positioning and configuration of the four (proximal, distal) spiral curls of the two stents were seen to remain essentially unchanged, from initial insertion and opening until the completion of the experiment. Mild ureter dilatation was noted, apparent by Day 5 and then essentially unchanged thereafter, in the region of the stent and somewhat proximal and distal to the spiral curls. Moreover, no delay of contrast excretion from the kidneys was observed; contrast excretion accumulated in the bladder, indicating no evidence of obstruction. Frequent checks of animal well-being by the veterinarian and veterinary staff confirmed that the pig exhibited no overt discomfort, disturbance, or abnormal behavior of any kind; the activity, appetite and sleep patterns of the pig remained unchanged, comparable to those known for healthy pigs, with normal weight gain throughout the entire duration of the experiment.
Upon completion of the weekly checks, on the final day of the experiment, visual inspection of the abdominal and thoracic cavities suggested no pathological findings; examination of the entire urinary tract, including the full length of the ureters and the interiors of the bladder and each renal pelvis, indicated no visible signs of inflammation, perforation, or hydronephrosis. Particular examination of the ureter wall in the vicinity of the of spiral curl ends of each stent also indicated normal anatomy (Figure 3); only mild ureteral dilatation was observed, as compared to observations in the initial pig cadaver test prior to stent insertion and as known relative to ureters from unstented pigs.
Detailed histological analysis of the tissue samples indicated that the kidney and bladder sections appeared entirely normal. The findings for both ureters were similar: minimal to mild eosinophilic inflammation, compatible with low-level irritation due to ureteral wall stretching, was present in all sections, but more pronounced in the dilated samples from vicinity of the spiral curl ends. Erosion, ulceration and infection were not identified. Furthermore, no stent encrustation was detected visually, noting that encrustation is not typically observed on stents in pigs.
Discussion
As noted in the Background, double-J ureteral stents and variations thereof cause substantial discomfort in 80% or more of patients, with significantly reduced quality of life, presumably due mostly to the present of the anchors in the bladder and kidney, as well as to urine reflux to the renal collecting system. High rates of lower urinary tract symptoms, flank pain and hematuria have been reported as a result of irritation by the foreign body and the reflux generated. Lack of peristalsis and excessive dilatation of the ureter have also been reported to be mechanisms affecting quality of life. As such, stent symptoms also have a high economic impact that might be underestimated. Costs caused by ureteral stenting are multifactorial. Work incapacity constitutes the major part of expenses, followed by outpatient/inpatient medical consultations and drug therapy due to stent-related symptoms.
While considerable literature offers evidence that the bladder (distal) anchor is a major factor affecting patient discomfort (5-9,11-13), literature regarding the degree of discomfort caused by the positioning of the proximal pigtail in the kidney is less clear. However, some literature focuses on identifying the degree to which pigtail positioning in the renal pelvis and different renal calyxes correlates to the intensity of patient discomfort (17,18), which also suggests that a proximal anchor placed essentially anywhere within the kidney may also be a source of patient discomfort.
In the context of distal anchors, “pigtail suture stents” and braided stents that eliminate the distal pigtail, and much or all of the mid-ureteral section of a stent—replacing these elements by a suture or braid—have been proposed (11-14). A recent patient trial showed promising results in terms of reduction in stent-related symptoms (13). Use of these stents has been reported for a range of treatments, including obstructing ureteral stones, ureteral strictures, extracorporeal shockwave lithotripsy, and for post-ureteroscopy treatment. It has been suggested, too, that replacing the distal section of a stent with a suture may reduce or limit reflux (12). Other efforts have focused on developing ureteral stents with specific anti-reflux properties (15). Note that the risk for infection posed by the presence of a suture tether in the YotiCurl stent can be considered no higher than with use of pigtail suture stents or conventional double-J stents, with or without a tether.
In the context of the existing literature and commercially-available ureteral stents, the YotiCurl stent design introduced here is novel in that it is fully intraureteral, eliminating both proximal and distal anchors in the bladder and kidney, and thus any possible direct irritation of anchors in the bladder or renal collecting system mucosa. Clearly, an animal model cannot yield specific information on discomfort in human clinical settings, but as reported above, the pig displayed no signs of overt discomfort, disturbance, or abnormal behavior of any kind, with activity, appetite and sleep patterns comparable to the behavior of the pig prior to stent insertion, as well as to patterns known for healthy pigs. Indeed, in terms of direct irritation, histology following the in vivo experiment confirmed normal mucosa both in the bladder near the ureterovesical junctions and in the renal pelvis. It might be speculated, too, that the lack of a bladder anchor may also lead to suppression of reflux and reduction of urinary symptoms, similar to the pigtail suture stents discussed above (12).
Given that the spiral curl design of the proximal and distal ends of the stent is intended to enable free urine drainage through both the ureteral and stent lumina, ureteral obstruction and/or hydronephrosis are less likely; this was confirmed, too, in the in vivo experiment.
Histology identified mild ureteral inflammation in the region of the stent curls: in this context, conventional double-J stents can cause ureteral dilatation and inflammation; similar inflammation in the bladder and/or renal pelvis caused by double-J stents is generally considered mild to moderate and usually resolves naturally following stent removal.
The YotiCurl stent is designed, principally, to maintain adequate urine drainage in a scenario of obstructing stone—prior to ureteroscopy to remove the stone—or ureteral stricture, wherein the spiral curls straddle the obstruction or stricture. In the case of obstructing stones, too, potential ureter dilatation by the stent and partial peristalsis may encourage mobilization of an obstructed stone downward to the ureterovesical junction, so that ureteroscopy may be even easier than if needed to treat at the proximal part of the ureter. For obstructions at the ureteropelvic junction, either by a stone or other etiology, the stent may still be used with its proximal curl placed within the renal pelvis and its distal part within the ureter, in a manner similar to other suggested stent designs that retain a kidney anchor but eliminate the distal anchor in the bladder (11-13). Another indication that might be considered could include pre-stenting of a ureter in preparation for complex retrograde intrarenal surgery, which may allow the use of larger access sheaths and easier extraction of stone fragments using baskets, where no concerns regarding ureterovesical junction narrowing exist.
The YotiCurl stent is not intended for treatment of post-operative edema at the level of the ureterovesical junction, nor is it intended for ureteral stones in the proximity of this junction where primary ureteroscopy can be performed easily if conservative treatment fails.
There are several limitations to this proof-of-concept in vivo study, in particular, noting that two ureters in a single animal model were tested, and limited variables could be evaluated. It is clear that a larger cohort of animal tests is required prior to generalizing the findings reported here. Moreover, ease of in vivo stent removal could not be confirmed because the stents remained within the ureters as required for the histological analysis, and changes in reflux behavior were not assessed. Finally, potential urinary symptoms (or reductions thereof), caused by irritation particularly in the ureterovesical junction, can only be evaluated directly in humans; similarly, other potential complications in the urinary system, such as strictures and obstruction, and stone migration, should be evaluated in human clinical trials.
Conclusions
This study presents a preliminary in vivo evaluation of the potential feasibility and safety of a newly-designed ureteral stent that resides completely within the ureter. An initial study testing placement of YotiCurl stents in two ureters in a single animal, as a proof-of-concept, confirmed straightforward endoscopic insertion, free urine drainage, no evident stent migration, mild ureteral dilatation and inflammation, no clearly observable hydronephrosis, and no changes in animal activity, mobility, appetite and sleep patterns.
Further testing on a suitably large number of animals, including control animals with conventional double-J stents and/or no stenting, is required to more fully evaluate stent function and all aspects of its impact on urinary tract function. Such testing can now be justified on the basis of this proof-of-concept study. Findings from this initial study cannot yet be generalized.
Acknowledgments
BB and YS appreciate the financial support of Yeda Research & Development Co., Ltd. and Mor Research Applications Ltd.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-41/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-41/dss
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-41/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-41/coif). YS and BB are co-inventors of a patent application (PCT/IB2021/052274), relating to aspects of this ureteral stent and/or its use, submitted jointly by Yeda Research & Development Co., Ltd. and Mor Research Applications Ltd., Israel. BB holds the Sam Zuckerberg Professorial Chair in Hydrology. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. 29-2021) granted by the Shamir Medical Center Institutional Animal Care and Use Committee (IACUC), in compliance with institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lundeen CJ, Forbes CM, Wong VKF, et al. Ureteral stents: the good the bad and the ugly. Curr Opin Urol 2020;30:166-70. [Crossref] [PubMed]
- Zimskind PD, Fetter TR, Wilkerson JL. Clinical use of long-term indwelling silicone rubber ureteral splints inserted cystoscopically. J Urol 1967;97:840-4. [Crossref] [PubMed]
- Finney RP. Experience with new double J ureteral catheter stent. J Urol 1978;120:678-81. [Crossref] [PubMed]
- Hepperlen TW, Mardis HK, Kammandel H. Self-retained internal ureteral stents: a new approach. J Urol 1978;119:731-4. [Crossref] [PubMed]
- Joshi HB, Stainthorpe A, MacDonagh RP, et al. Indwelling ureteral stents: evaluation of symptoms, quality of life and utility. J Urol 2003;169:1065-9; discussion 1069. [Crossref] [PubMed]
- Damiano R, Autorino R, De Sio M, et al. Does the size of ureteral stent impact urinary symptoms and quality of life? A prospective randomized study. Eur Urol 2005;48:673-8. [Crossref] [PubMed]
- Miyaoka R, Monga M. Ureteral stent discomfort: Etiology and management. Indian J Urol 2009;25:455-60. [Crossref] [PubMed]
- Al-Aown A, Kyriazis I, Kallidonis P, et al. Ureteral stents: new ideas, new designs. Ther Adv Urol 2010;2:85-92. [Crossref] [PubMed]
- Ramachandra M, Mosayyebi A, Carugo D, et al. Strategies to Improve Patient Outcomes and QOL: Current Complications of the Design and Placements of Ureteric Stents. Res Rep Urol 2020;12:303-14. [Crossref] [PubMed]
- Forbes C, Scotland KB, Lange D, et al. Innovations in Ureteral Stent Technology. Urol Clin North Am 2019;46:245-55. [Crossref] [PubMed]
- Ponsot Y, Sawhney S, Carmel M. A simple alteration in a ureteral double J stent to improve its clinical acceptability. Prog Urol 1994;4:420-2. [PubMed]
- Vogt B, Desgrippes A, Desfemmes FN. Changing the double-pigtail stent by a new suture stent to improve patient's quality of life: a prospective study. World J Urol 2015;33:1061-8. [Crossref] [PubMed]
- Bosio A, Alessandria E, Agosti S, et al. Pigtail Suture Stents Significantly Reduce Stent-related Symptoms Compared to Conventional Double J Stents: A Prospective Randomized Trial. Eur Urol Open Sci 2021;29:1-9. [Crossref] [PubMed]
- Lim KS, Law ZW, Chow MWL, et al. Peak stent discomfort occurs early and ureteral stent with distal loop design has less pain-A pilot prospective randomised single-blinded trial over 2 weeks. Asian J Urol 2020;7:357-62. [Crossref] [PubMed]
- Soria F, Morcillo E, Serrano A, et al. Evaluation of a New Design of Antireflux-biodegradable Ureteral Stent in Animal Model. Urology 2018;115:59-64. [Crossref] [PubMed]
- Yoshida T, Inoue T, Taguchi M, et al. Efficacy and Safety of Complete Intraureteral Stent Placement versus Conventional Stent Placement in Relieving Ureteral Stent Related Symptoms: A Randomized, Prospective, Single Blind, Multicenter Clinical Trial. J Urol 2019;202:164-70. [Crossref] [PubMed]
- Liatsikos EN, Gershbaum D, Kapoor R, et al. Comparison of symptoms related to positioning of double-pigtail stent in upper pole versus renal pelvis. J Endourol 2001;15:299-302. [Crossref] [PubMed]
- El-Nahas AR, El-Assmy AM, Shoma AM, et al. Self-retaining ureteral stents: analysis of factors responsible for patients' discomfort. J Endourol 2006;20:33-7. [Crossref] [PubMed]




