
Real-world data on the efficacy and safety of pazopanib in IMDC favorable- and intermediate-risk metastatic renal cell carcinoma: a multicenter retrospective cohort study of Chinese patients
Introduction
Renal cell carcinoma (RCC) is one of the most common malignant tumors of the urinary system, accounting for about 5% and 3% of all new adult male and female cancer cases, respectively (1). Clear cell RCC (ccRCC) is the most common and highly malignant pathological type of RCC, accounting for 70–85% of all patients with renal cancer. Approximately 25–30% of patients with ccRCC have metastases at first diagnosis (2). The prognosis of metastatic RCC (mRCC) can be predicted according to the risk stratification of the International Metastatic RCC Database Consortium (IMDC) (3,4). Previous studies have indicated that 17–23% of patients with mRCC are in the IMDC favorable-risk group, while approximately 52% of patients are in the intermediate-risk group (5,6). With no standard predictive biomarker available to aid in therapy selection, the current individualized treatment of patients with mRCC relies on validated prognostic risk models.
In recent years, the combination of tyrosine kinase inhibitors (TKI) and immune checkpoint inhibitors (ICPIs), including anti–programmed cell death-1 (PD-1) antibody, anti–programmed death ligand 1 (PD-L1) antibody, and anti–cytotoxic T-lymphocyte–associated antigen 4 (CTLA4) antibody, has significantly improved the prognosis of mRCC (7-9). Several clinical trials have confirmed the advantages of novel regimens of vascular endothelial factor receptor (VEGFR) inhibitor monotherapy in first-line treatment (10,11). According to the National Comprehensive Cancer Network (NCCN) guidelines, pazopanib, sunitinib, and axitinib plus pembrolizumab (anti-PD-1) are now recommended as the first-line treatment of patients with IMDC favorable-risk mRCC, while ipilimumab (anti-CTLA4) plus nivolumab (anti-PD-1) or axitinib plus pembrolizumab are indicated in the first-line treatment of patients with intermediate risk (12). However, the outcomes of different treatment options are highly heterogeneous, and the individualized selection of the best first-line option is essential for the treatment of mRCC.
The CheckMate-214 study compared the efficacy of PD-1 plus CTLA4 antibody and VEGFR inhibitor in the first-line treatment of patients stratified by IMDC risk group (13). Combination therapy with nivolumab plus ipilimumab was superior over sunitinib in patients in the IMDC intermediate- or poor-risk groups, but sunitinib yielded better outcomes in favorable-risk patients (13). In the phase III KEYNOTE-426 and KEYNOTE-581/CLEAR studies, pembrolizumab plus axitinib and pembrolizumab plus lenvatinib were not superior to sunitinib with respect to overall survival (OS) in patients in the IMDC favorable-risk subgroup (14,15). Based on the current research results, the recommended treatment for IMDC intermediate- and poor-risk patients is a VEGFR inhibitor plus a PD-1/PD-L1 antibody, or nivolumab plus ipilimumab. However, due to the economic burden of long-term medication and health insurance policies, patients with IMDC favorable risk, especially in China, are still recommended targeted agent monotherapy such as sunitinib and pazopanib. Besides, although previous studies have provided the survival benefits and safety of pazopanib targeted therapy, due to the strictly selected patients has poor external validation and the clinical characteristics are complex in real-world clinical practice, it is necessary to study the real-world evidence to provide insight into the effectiveness and tolerability of pazopanib in clinical practice, which can be contrasted with the more selected patient populations entering prospective clinical trials.
The treatment options for IMDC intermediate-risk patients are the most controversial. Among these patients, those with 1 risk factor may be more suitable for targeted agent monotherapy, while patients with 2 risk factors may obtain better survival benefits from nivolumab plus ipilimumab. Indeed, the CheckMate-214 study also suggested that intermediate-risk patients be further stratified to more accurately predict treatment outcomes (13). Several previous studies have reported survival differences between patients with IMDC 1 vs. 2 risk factors (16-19). However, previous first-line randomized controlled studies used sunitinib as the standard treatment option, and data on the treatment of IMDC favorable- and intermediate-risk patients with pazopanib are lacking.
To this end, this study aimed to illustrate the survival benefits and drug safety of single pazopanib treatment using real-world data of patients with IMDC favorable- and intermediate-risk mRCC. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-312/rc).
Methods
Patients
This was a retrospective, multicenter, cohort study of 143 patients with mRCC from 3 independent cancer centers in China who received pazopanib targeted therapy between May 2017 and February 2020. The clinicopathological data of the patients were collected from the medical records of each center. Considering the adequate sample size and long-term follow-up period, all patients with pazopanib targeted therapy from 3 independent cancer centers from May 2017 to February 2020 were included in the candidate study population and the sample size was determined according to inclusion and exclusion criteria. All patients had been treated in a ‘real-life’ setting outside clinical trials and received their first dose of pazopanib according to their own tolerance. Patients were treated with pazopanib 800 mg (n=54) or 600 mg (n=89) orally once daily until disease progression, occurrence of unacceptable toxicity, or death. Dose modification or discontinuation was administered according to the patient’s tolerance. The inclusion criteria were as follows: (I) patients were aged ≥18 years; (II) patients had a histological or cytological diagnosis of RCC (either ccRCC or non-ccRCC) and had radiologically measurable metastatic disease; (III) patients had an IMDC risk stratification of favorable or intermediate risk; and (IV) patients received pazopanib as a first-line treatment. The exclusion criteria were as follows: (I) patients received first-line targeted therapies other than pazopanib; (II) patients received neoadjuvant therapy; (III) patients had an IMDC risk stratification of poor risk; and (IV) patients had no clear progression time and relapse/metastasis date. According to the guidelines, targeted agent monotherapy is not recommended for IMDC poor-risk patients, so our study did not include these patients. In addition, 6 patients on drugs other than pazopanib as a first-line targeted therapy and 11 patients on neoadjuvant treatment were excluded. After removing 16 IMDC poor-risk patients, a total of 143 IMDC favorable- and intermediate-risk patients with mRCC who received pazopanib as a first-line treatment were enrolled in our study.
The patients were classified as IMDC favorable, intermediate, or poor risk if they had 0, 1 or 2, or ≥3 of the following risk factors: (I) time from initial diagnosis to initiation of therapy <1 year; (II) Karnofsky Performance Status (KPS) <80%; (III) serum hemoglobin level < lower limit of normal (LLN); (IV) serum corrected calcium level > upper limit of normal (ULN); (V) absolute neutrophil count > ULN; and (VI) platelet count > ULN. Laboratory test results were standardized against institutional ULN and LLN values when appropriate. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Fudan University Shanghai Cancer Center (Ethical IRB No. 050432-4-1911D, Shanghai, China). All patients participating in this study signed informed consent forms.
Assessment
The primary endpoint was progression-free survival (PFS), which was defined as the period between the date of commencement of first-line pazopanib treatment and the date of discontinuation of the treatment due to disease progression or death from any cause. Other study objectives included overall response rate (ORR); safety; and correlation among PFS and several factors, including age, IMDC risk stratification and factors, metastatic information, and local treatment history.
Patient responses were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 by 3 authors. The dose of the drug was determined by the treating physician according to the patient’s condition. The timing of assessments was at the discretion of the treating physician and usually occurred once every 3 months. The clinical follow-up included history taking, physical examination, and biochemistry test every 2 to 4 weeks, and radiological imaging test every 3 months. For patients who lost to clinical follow up, telephone interviews or online follow-up were performed to confirm the survival status and other follow-up information.
Treatment-related adverse drug reactions (ADRs) were recorded by each physician according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0. Patients with grade 1 ADRs were managed symptomatically without lowering the dose or withdrawing the drug. If grade 2 ADRs occurred, patients were required to reduce the drug dose until the ADRs resolved to ≤ grade 1. If grade 3 ADRs occurred, pazopanib was withheld until the ADRs decreased to ≤ grade 1.
Statistical analysis
Continuous variables were reported as means ± standard deviation (SD), medians, and ranges; categorical variables were reported as number and percentage of the total population. To account for some missing data responses, we performed multiple imputations by intervention assignment. We used the multivariable imputation by chained equations procedure, creating 5 imputed datasets and combing regression results. Evaluations were based on point estimates and 95% CIs.
In the descriptive analyses of PFS, the cumulative probability of being event-free at each time in the whole study population, as well as in each subgroup, was computed using the Kaplan–Meier product limit estimator. Kaplan–Meier curves in the different classes of each factor were compared with the log-rank test. To assess the relative prognostic role of each factor, while adjusting for other factors, a series of univariate and multivariate Cox models were fitted to the data with PFS as the dependent variable. Variables which had significant prognostic value in the univariate Cox regression were included in the final multivariate Cox regression analysis, and variables with P<0.05 in both univariate and multivariate Cox regression analyses were identified as independent prognostic factors.
The proportions of patients showing overall responses were computed and compared in different classes of each factor using the chi-square test for heterogeneity or the chi-square test for trend, as appropriate.
Statistical software used in this study was RStudio of version 1.3 and the cutoff for P-value of statistical significance was defined at 0.05.
Results
Clinicopathological features
The data of 143 patients with IMDC favorable- and intermediate-risk mRCC treated by pazopanib as a first-line therapy were collected from 3 independent cancer centers. The overall characteristics of the patients are summarized in Table 1.
Table 1
| Characteristics | Overall (N=143) | Favorable risk (N=46) | Intermediate risk (N=97) |
|---|---|---|---|
| Center, n (%) | |||
| FUSCC | 75 (52.4) | 23 (50.0) | 52 (53.6) |
| FMUUH | 38 (26.6) | 12 (26.1) | 26 (26.8) |
| HMUTH | 30 (21.0) | 11 (23.9) | 19 (19.6) |
| Gender, n (%) | |||
| Male | 106 (75.5) | 36 (78.3) | 70 (72.2) |
| Female | 37 (25.9) | 10 (21.7) | 27 (27.8) |
| Age | |||
| N | 142 | 46 | 96 |
| Mean (SD) | 58.19 (11.12) | 59.41 (9.52) | 57.60 (11.81) |
| Median (Q1, Q3) | 59.50 (51.25, 66.00) | 60.00 (54.50, 66.50) | 59.00 (50.75, 66.00) |
| Min, Max | 25.00–81.00 | 30.00–79.00 | 25.00–81.00 |
| Missing, n (%) | 1 (0.7) | 0 (0) | 1 (1.0) |
| Pathological type, n (%) | |||
| ccRCC | 120 (83.9) | 42 (91.3) | 78 (80.4) |
| Non-ccRCC | 15 (10.5) | 1 (2.2) | 14 (14.4) |
| Missing | 8 (5.6) | 3 (6.5) | 5 (5.2) |
| Maximum tumor size (cm) | |||
| N | 99 | 27 | 72 |
| Mean (SD) | 6.97 (2.57) | 6.84 (2.11) | 7.01 (2.74) |
| Median (Q1, Q3) | 7.00 (4.95, 9.00) | 7.00 (5.10, 7.90) | 7.00 (4.80, 9.00) |
| Range | 1.00–13.00 | 3.00–12.00 | 1.00–13.00 |
| <7, n (%) | 47 (32.9) | 13 (28.3) | 34 (35.0) |
| ≥7, n (%) | 52 (36.4) | 14 (30.4) | 38 (39.2) |
| Missing, n (%) | 44 (30.8) | 19 (41.3) | 25 (25.8) |
| Laterality, n (%) | |||
| Bilateral | 1 (0.7) | 0 (0) | 1 (1.0) |
| Right | 71 (49.7) | 19 (41.3) | 52 (53.6) |
| Left | 68 (47.6) | 26 (56.5) | 42 (43.3) |
| Missing | 3 (2.1) | 1 (2.2) | 2 (2.1) |
| IMDC risk factors, n (%) | |||
| 0 | 46 (32.2) | 46 (100.0) | 0 (0) |
| 1 | 60 (37.7) | 0 (0) | 60 (61.9) |
| 2 | 37 (23.3) | 0 (0) | 37 (38.1) |
| Location of metastatic sites, n (%) | |||
| NA | 10 (7.0) | 2 (4.3) | 8 (8.2) |
| Single metastasis, n (%) | |||
| Lung | 44 (30.8) | 20 (43.5) | 24 (24.7) |
| Bone | 9 (6.3) | 2 (4.3) | 7 (7.2) |
| Others | 19 (13.2) | 5 (10.9) | 14 (14.4) |
| Multiple metastases, n (%) | |||
| Including lung | 44 (30.8) | 14 (30.4) | 30 (30.9) |
| Excluding lung | 17 (11.9) | 3 (6.5) | 14 (14.4) |
| Baseline number of R/M organs, n (%) | |||
| 0 | 10 (7.0) | 2 (4.3) | 8 (8.2) |
| 1 | 72 (50.3) | 27 (58.7) | 45 (46.4) |
| 2 | 39 (27.3) | 15 (32.6) | 24 (24.7) |
| ≥3 | 22 (15.4) | 2 (4.3) | 20 (20.6) |
| Partial treatment, n (%) | |||
| No partial treatment | 100 (69.9) | 31 (67.4) | 69 (71.1) |
| Primary site resection | 14 (9.8) | 3 (6.5) | 11 (11.3) |
| Metastasis site resection | 22 (15.4) | 9 (19.6) | 13 (13.4) |
| Missing | 7 (4.9) | 3 (6.5) | 4 (4.1) |
IMDC, International Metastatic renal cell carcinoma Database Consortium; FUSCC, Fudan University Shanghai Cancer Center; FMUUH, Fujian Medical University Union Hospital; HMUTH, Harbin Medical University Tumor Hospital; SD, standard deviation; ccRCC, clear cell renal cell carcinoma; R/M, relapse/metastasis; NA, not available.
The mean age of the study cohort was 58.19±11.12 years, and there were 106 males (74.1%) and 37 females (25.9%). ccRCC was the most common histological type, accounting for 83.9% (120/143) of patients. In addition, there were 10.5% (15/143) patients with non-ccRCC and 5.6% (8/143) patients with unclassified RCC. The mean size of the primary kidney tumor was 6.97±2.57 cm, with 52 (36.4%) cases lager than 7 cm and 47 (32.9%) smaller than 7 cm.
Based on IMDC risk stratification, 46 (32.2%) patients had a favorable prognosis, and 97 (67.8%) patients had an intermediate prognosis. In the intermediate-risk group, 60 (61.9%) patients had 1 risk factor, and 37 (38.1%) patients had 2 risk factors. The demographic, disease, and clinical characteristics of the patients analyzed by IMDC and Memorial Sloan Kettering Cancer Center (MSKCC) stratification are summarized in Table 1 and supplementary Table 1.
Imaging examination and puncture biopsy confirmed 44 (30.8%) cases of lung metastasis, 9 (6.3%) cases of bone metastasis, and 21 (13.2%) cases of other single organ metastasis. In addition, 44 (30.8%) patients had multiple organ metastases including lung metastasis, while 17 (11.9%) patients had multiple organ metastases that did not include lung metastasis.
In the follow-up treatment, 100 (69.9%) patients did not receive local treatment for the metastatic lesions, 14 (9.8%) patients underwent primary site resection, and 22 (15.4%) patients received surgical resection of the metastatic lesions.
The hematological indexes of the patients before and 3 months after targeted treatment were analyzed. Granulocytes (P<0.001), platelet (P<0.001) and hemoglobin (P=0.009) were significantly decreased 3 months after treatment, but creatine (P<0.001), blood urea nitrogen (BUN; P=0.028) and eosinophil (P<0.001) was significantly increased 3 months after treatment (Table 2).
Table 2
| Index | Before target targeted (N=143) | 3 months after targeted therapy (N=143) | Change from baseline (N=143) | P value* |
|---|---|---|---|---|
| GRAN | 0.001 | |||
| Mean (SD) | 3.64 (1.53) | 3.25 (1.12) | −0.39 (1.40) | |
| Median (Q1, Q3) | 3.53 (2.50, 4.40) | 3.20 (2.53, 3.70) | −0.24 (−0.80, 0.30) | |
| Min, Max | 0.50, 10.20 | 0.50, 8.10 | −7.00, 4.34 | |
| Lymphocyte | 0.65 | |||
| Mean (SD) | 1.80 (1.43) | 1.74 (0.74) | −0.54 (1.42) | |
| Median (Q1, Q3) | 1.58 (1.10, 2.10) | 1.58 (1.20, 2.20) | −0.05 (−0.28, 0.40) | |
| Min, Max | 0.18, 16.40 | 0.26, 3.37 | −15.00, 2.47 | |
| Eosinophil | <0.001 | |||
| Mean (SD) | 0.14 (0.15) | 0.26 (0.40) | 0.12 (0.38) | |
| Median (Q1, Q3) | 0.10 (0.05, 0.18) | 0.14 (0.08, 0.23) | 0.02 (−0.02, 0.09) | |
| Min, Max | 0.00, 1.40 | 0.00, 2.08 | −0.75, 1.91 | |
| PLT | <0.001 | |||
| Mean (SD) | 215.52 (71.76) | 197.89 (65.77) | −17.64 (46.66) | |
| Median (Q1, Q3) | 207.00 (161.00, 261.00) | 185.00 (152.00, 242.00) | −10.00 (−40.00, 8.00) | |
| Min, Max | 43.00, 460.00 | 60.00, 398.00 | −196.00, 101.00 | |
| Hemoglobin | 0.009 | |||
| Mean (SD) | 133.17 (21.21) | 129.89 (21.07) | −3.28 (14.79) | |
| Median (Q1, Q3) | 132.00 (118.00, 151.00) | 128.00 (117.00, 145.00) | −4.00 (−12.00, 4.00) | |
| Min, Max | 78.00, 188.00 | 84.00, 185.00 | −39.00, 54.00 | |
| LDH | 0.158 | |||
| Mean (SD) | 211.12 (124.02) | 228.55 (242.75) | 17.43 (146.83) | |
| Median (Q1, Q3) | 183.00 (158.00, 219.00) | 187.00 (166.00, 213.00) | 5.00 (−20.00, 34.00) | |
| Min, Max | 110.00, 1,424.00 | 100.00, 3,000.00 | −317.00, 1,576.00 | |
| Calcium | 0.235 | |||
| Mean (SD) | 2.26 (0.18) | 2.25 (0.21) | −0.01 (0.12) | |
| Median (Q1, Q3) | 2.23 (2.19, 2.40) | 2.25 (2.10, 2.36) | 0.00 (−0.10, 0.08) | |
| Min, Max | 1.18, 2.75 | 1.60, 2.96 | −0.43, 0.31 | |
| Creatine | <0.001 | |||
| Mean (SD) | 90.90 (30.41) | 97.25 (33.51) | 6.35 (21.17) | |
| Median (Q1, Q3) | 88.00 (70.00, 103.00) | 95.00 (74.00, 110.00) | 4.00 (−5.00, 14.00) | |
| Min, Max | 40.00, 260.00 | 47.00, 356.00 | −49.00, 126.00 | |
| BUN | 0.028 | |||
| Mean (SD) | 6.01 (2.05) | 6.27 (1.96) | 0.26 (1.41) | |
| Median (Q1, Q3) | 5.60 (4.58, 7.27) | 5.58 (3.90, 7.40) | 0.20 (−0.47, 0.79) | |
| Min, Max | 1.92, 15.50 | 3.10, 14.10 | −3.54, 5.54 | |
| Urine protein | 0.071 | |||
| Mean (SD) | 0.28 (0.65) | 0.33 (0.63) | 0.05 (0.32) | |
| Median (Q1, Q3) | 0.00 (0.00, 0.00) | 0.00 (0.00, 1.00) | 0.00 (0.00, 0.00) | |
| Min, Max | 0.00, 3.00 | 0.00, 3.00 | −1.00, 1.00 |
*, paired t-test. GRAN, Granulocytes; SD, standard deviation; PLT, platelet; LDH, lactate dehydrogenase; BUN, blood urea nitrogen.
Effectiveness: PFS
A total of 103 patients received only first-line targeted treatment with pazopanib. Among these patients, 66 were under treatment, and 37 had discontinued treatment. Thirty-four patients switched to second-line targeted therapy. Among these patients, 24 were treated with axitinib, 6 were treated with everolimus, 3 were treated with sorafenib, and 1 received sunitinib treatment. Furthermore, 6 patients switched to third-line targeted treatment after the failure of second-line therapy (Table S1).
The median follow-up time was 24.7 months, and the median PFS was 21.2 months (95% CI, 17.19–27.14; Figure 1). The PFS of patients in the IMDC favorable-risk group was significantly better than that of patients in the IMDC intermediate-risk group, with a median PFS of 27.1 months and 17.2 months, respectively (P=0.0019; Figure 2A). In the intermediate-risk group, PFS was much longer in patients with 1 risk factor than in those with 2 risk factors, with a median PFS of 25.9 vs. 11.2 months, respectively (P<0.0001; Figure 2B). Patients with lung metastasis only had longer PFS than those with bone metastasis only (median 25.9 vs. 21.2 months, respectively). Patients with multiple metastases including lung metastasis had longer PFS than patients with multiple metastases without lung metastasis (median 31.0 vs. 11.21 months, respectively; P=0.0051; Figure 3A). Patients with a single metastatic organ at baseline had longer PFS than patients with multiple metastases at the time of diagnosis (P=0.0009; Figure 3B), while patients with 2 metastatic organs had better PFS than patients with 3 or more metastatic organs (P=0.000; Figure 3C). When pazopanib effectiveness was assessed in patients aged ≥65 and <65 years, the survival profile was generally similar between the 2 age groups (Figure 3D). PFS was longer in patients who received primary kidney tumor resection, and local therapy for the metastatic site seemed to benefit patients in the IMDC favorable-risk group but not those in the IMDC intermediate-risk group (Figure 4A,4B). Survival details are summarized in Table 3. To identify potential prognostic factors, univariate and multivariate Cox proportional hazard models for overall PFS were performed. Male gender (P=0.018), higher IMDC risk factors (P=0.005), and a higher baseline number of relapsed/metastatic organs (P=0.004) were significantly correlated with poor PFS in both the univariate and multivariate Cox proportional hazard models, which further verified our conclusions. In addition, abnormal eosinophil count (P=0.007), abnormal hemoglobin (P=0.023), and abnormal lactate dehydrogenase (LDH; P=0.011) were significantly correlated with poor outcomes in the univariate Cox regression model but not in the multivariate Cox model (Table 4).
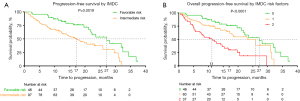
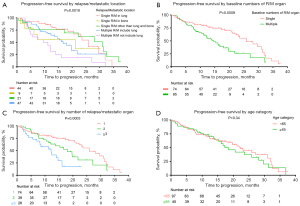

Table 3
| Characteristic | Median survival (95% CI) |
|---|---|
| Overall | 21.16 (17.19–27.14) |
| IMDC risk groups | |
| Favorable | 27.14 (21.82–NA) |
| Intermediate | 17.19 (12.69–25.88) |
| IMDC risk factors | |
| 0 | 27.14 (21.82–NA) |
| 1 | 25.88 (17.19–NA) |
| 2 | 11.21 (7.17–19.74) |
| Baseline R/M: location | |
| Single lung metastasis | 25.88 (23.07–NA) |
| Single bone metastasis | 21.16 (6.15–NA) |
| Multiple metastases including lung | 17.98 (13.69–NA) |
| Multiple metastases excluding lung | 11.21 (5.59–NA) |
| Single metastasis excluding lung and bone | 31.01 (22.28–NA) |
| Baseline number of R/M organs | |
| 1 | 25.92 (23.07–31.93) |
| 2 | 17.98 (12.07–NA) |
| ≥3 | 12.30 (8.53–NA) |
| Age category | |
| <65 years | 22.28 (17.98–31.93) |
| ≥65 years | 18.45 (15.34–29.65) |
| Local treatment | |
| No | 19.67 (16.10–25.92) |
| Primary site | 31.01 (23.07–NA) |
| Metastasis site | 15.37 (9.52–NA) |
| Number of R/M location | |
| No R/M | 12.15 (5.02–NA) |
| Single | 25.92 (23.07–31.93) |
| Multiple | 14.64 (12.07–27.14) |
| Treatment response | |
| SD | 19.67 (16.10–28.10) |
| PD | 2.20 (1.98–8.20) |
| PR | 31.01 (25.92–NA) |
PFS, progression free survival; IMDC, International Metastatic renal cell carcinoma Database Consortium; R/M, relapse/metastasis; SD, stable disease; PD, progressive disease; PR, partial response; CI, confidence interval.
Table 4
| Variable | N | Event N | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI1 | P value | ||||
| Gender | 0.022 | 0.018 | |||||||
| Male | 106 | 62 | – | – | – | – | |||
| Female | 37 | 15 | 0.53 | 0.30–0.94 | 0.41 | 0.19–0.89 | |||
| Age category | 0.563 | ||||||||
| <65 years | 97 | 49 | – | – | |||||
| ≥65 years | 45 | 27 | 1.15 | 0.72–1.84 | |||||
| Pathological type | 0.328 | ||||||||
| ccRCC | 120 | 63 | – | – | |||||
| Non-ccRCC | 15 | 8 | 1.48 | 0.70–3.12 | |||||
| Maximum tumor size (cm) | 0.330 | ||||||||
| <7 | 47 | 27 | – | – | |||||
| ≥7 | 52 | 28 | 0.77 | 0.45–1.31 | |||||
| IMDC risk factors | <0.001 | 0.005 | |||||||
| 0 | 46 | 21 | – | – | – | – | |||
| 1 | 60 | 28 | 1.69 | 0.92–3.10 | 20.4 | 3.76–110.00 | |||
| 2 | 37 | 28 | 4.03 | 2.16–7.52 | 16.7 | 3.38–82.40 | |||
| Local treatment | 0.112 | ||||||||
| No | 100 | 54 | – | – | |||||
| Yes | |||||||||
| Primary site | 14 | 4 | 0.39 | 0.14–1.08 | |||||
| Metastasis site | 22 | 17 | 0.94 | 0.53–1.68 | |||||
| Baseline relapse/metastasis | 0.012 | ||||||||
| No | 10 | 6 | – | – | |||||
| Yes—single metastasis | |||||||||
| Lung | 44 | 18 | 0.42 | 0.17–1.07 | |||||
| Bone | 9 | 5 | 0.77 | 0.24–2.55 | |||||
| Others | 19 | 11 | 0.34 | 0.12–0.98 | |||||
| Yes—multiple metastases | |||||||||
| Including lung | 44 | 22 | 0.72 | 0.29–1.79 | |||||
| Excluding lung | 17 | 15 | 1.3 | 0.50–3.37 | |||||
| Number of R/M location | 0.007 | ||||||||
| No relapse/metastasis | 10 | 6 | – | – | |||||
| Single | 72 | 34 | 0.42 | 0.18–1.03 | |||||
| Multiple | 61 | 37 | 0.88 | 0.37–2.10 | |||||
| Baseline number of R/M organs | 0.008 | 0.004 | |||||||
| 0 | 10 | 6 | – | – | – | – | |||
| 1 | 72 | 34 | 0.42 | 0.17–1.02 | 0.17 | 0.05–0.53 | |||
| 2 | 39 | 23 | 0.75 | 0.30–1.85 | 0.39 | 0.12–1.27 | |||
| ≥3 | 22 | 14 | 1.24 | 0.47–3.24 | 0.55 | 0.16–1.87 | |||
| Baseline GRAN | 0.214 | ||||||||
| Normal | 114 | 61 | – | – | |||||
| Abnormal | 7 | 5 | 1.89 | 0.75–4.77 | |||||
| Baseline lymphocyte | 0.245 | ||||||||
| Normal | 99 | 55 | – | – | |||||
| Abnormal | 22 | 11 | 1.5 | 0.78–2.91 | |||||
| Baseline eosinophil | 0.007 | 0.736 | |||||||
| Normal | 103 | 51 | – | – | – | – | |||
| Abnormal | 18 | 15 | 2.38 | 1.32–4.27 | 1.16 | 0.49–2.71 | |||
| Baseline PLT | 0.489 | ||||||||
| Normal | 104 | 52 | – | – | |||||
| Abnormal | 17 | 14 | 1.24 | 0.68–2.26 | |||||
| Baseline hemoglobin | 0.023 | 0.669 | |||||||
| Normal | 83 | 39 | – | – | – | – | |||
| Abnormal | 38 | 27 | 1.8 | 1.10–2.97 | 1.19 | 0.54–2.59 | |||
| Baseline LDH | 0.011 | 0.598 | |||||||
| Normal | 80 | 37 | – | – | – | – | |||
| Abnormal | 41 | 29 | 1.94 | 1.17–3.20 | 0.82 | 0.39–1.72 | |||
| Baseline calcium | 0.512 | ||||||||
| Normal | 90 | 50 | – | – | |||||
| Abnormal | 31 | 16 | 1.21 | 0.69, 2.15 | |||||
| Baseline creatine | 0.169 | ||||||||
| Normal | 93 | 49 | – | – | |||||
| Abnormal | 28 | 17 | 1.5 | 0.86, 2.64 | |||||
| Baseline BUN | 0.968 | ||||||||
| Normal | 87 | 46 | – | – | |||||
| Abnormal | 34 | 20 | 0.99 | 0.58, 1.68 | |||||
| Urine protein | 0.379 | ||||||||
| Normal | 101 | 58 | – | – | |||||
| Abnormal | 19 | 7 | 0.71 | 0.32–1.57 | |||||
PFS, progression-free survival; ccRCC, clear cell renal cell carcinoma; IMDC, International Metastatic renal cell carcinoma Database Consortium; HR, hazard ratio; R/M, relapse/metastasis; GRAN, Granulocytes; PLT, platelet; LDH, lactate dehydrogenase; BUN, blood urea nitrogen; CI, confidence interval.
Antitumor activity: response rate
According to the radiology review, the best response for pazopanib treatment was 40/140 (29%) partial response (PR), 86/140 (61%) stable disease (SD), and 14/140 (10%) progressive disease (PD). The response rate was significantly associated with IMDC risk stratification, being 20/46 (43%) in the favorable-risk group and 20/94 (21%) in the intermediate-risk group (P=0.007). Additionally, low IMDC risk factors were significantly correlated with the best response rate (P=0.027; Table 5). A waterfall plot revealed the changes in tumor size by treatment response and IMDC risk stratification (Figure 4C,4D).
Table 5
| Characteristic | Response rate | OR2 | 95% CI | P value |
|---|---|---|---|---|
| Treatment response | ||||
| SD | 86/140 (61%) | |||
| PD | 14/140 (10%) | |||
| PR | 40/140 (29%) | |||
| IMDC risk groups | 0.007 | |||
| Favorable | 20/46 (43%) | – | – | |
| Intermediate | 20/94 (21%) | 0.35 | 0.16–0.75 | |
| IMDC risk factors | 0.027 | |||
| 0 | 20/46 (43%) | – | – | |
| 1 | 12/57 (21%) | 0.35 | 0.14–0.81 | |
| 2 | 8/37 (22%) | 0.36 | 0.13–0.93 |
ORR, overall response rate; OR, odds ratio; SD, stable disease; PD, progressive disease; PR, partial response; IMDC, International Metastatic renal cell carcinoma Database Consortium; CI, confidence interval.
Safety
ADRs (all grades) were reported in 65 patients (45.5%). The most common ADRs were change in hair color (47.7%), hypertension (40.0%), diarrhea (40.0%), proteinuria (38.5%), elevation of transaminase (35.4%), and hand–foot skin reaction (32.3%). In addition, neutrocytopenia (12.3%), rash (9.2%), thrombocytopenia (10.8%), hypothyroidism (7.7%), and anemia (6.2%) were also reported (Table 6).
Table 6
| ADR | All (N=143) | Grade | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Any ADR | 65/143 (45.5%) | 0 | 0 | 0 |
| Elevation of transaminase | 23/65 (35.4%) | 12/23 (52.2%) | 7/23 (30.4%) | 4/23 (17.4%) |
| Changes in hair color | 31/65 (47.7%) | 29/31 (93.5%) | 2/31 (6.5%) | 0 |
| Hand–foot skin reaction | 21/65 (32.3%) | 9/21 (42.9%) | 8/21 (38.1%) | 4/21 (19.0%) |
| Rash | 6/65 (9.2%) | 5/6 (83.3%) | 0 | 1/6 (16.7%) |
| Hypertension | 26/65 (40.0%) | 7/26 (26.9%) | 14/26 (53.8%) | 5/26 (19.2%) |
| Diarrhea | 26/65 (40.0%) | 14/26 (53.8%) | 8/26 (30.8%) | 4/26 (15.4%) |
| Neutrocytopenia | 8/65 (12.3%) | 2/8 (25.0%) | 4/8 (50.0%) | 2/8 (25.0%) |
| Thrombocytopenia | 7/65 (10.8%) | 4/7 (57.1%) | 3/7 (42.9%) | 0 |
| Anemia | 4/65 (6.2%) | 2/4 (50.0%) | 2/4 (50.0%) | 0 |
| Proteinuria | 25/65 (38.5%) | 14/25 (56.0%) | 8/25 (32.0%) | 3/25 (12.0%) |
| Hypothyroidism | 5/65 (7.7%) | 5/5 (100.0%) | 0 | 0 |
ADR, adverse drug reaction.
Discussion
This real-world data analysis found that patients in the IMDC favorable-risk group had the best prognosis and drug response, and patients in the intermediate-risk group with 1 risk factor had better PFS than those with 2 risk factors. Pazopanib was most suitable for patients with lung metastasis only, and local treatment for metastatic lesions might only be effective for patients in the IMDC favorable-risk group. The most common ADRs of pazopanib were change in hair color, hypertension, diarrhea, proteinuria, elevation of transaminase, and hand–foot skin reaction. Therefore, our research further clarified the population who can benefit from pazopanib targeted therapy and provided precise and individualized treatment strategies.
The treatment landscape for mRCC is changing rapidly, and phase 3 clinical trials with different combinations of available therapies have presented unexpected results. New treatment options have recently been recommended based on different IMDC risk groups (20,21). As indicated in the Checkmate-214 trial, different efficacy outcomes in patients treated with nivolumab plus ipilimumab occur between different risk groups (21). Moreover, several previous studies have demonstrated survival differences between patients with 1 versus 2 risk factors (16-18,22). However, at present, it is unknown whether the treatment response of pazopanib is consistent across patients with IMDC favorable- and intermediate-risk groups or with different metastatic sites. As pazopanib has shown objective efficacy in some patients, clarifying the most appropriate and specific population for pazopanib treatment may further improve the efficacy and safety of the treatment.
In addition to the treatment of patients with elderly or frail or poor prognosis, the favorable safety of pazopanib has made this agent appealing for the treatment of young patients with good physical status and prognostic characteristics or those needing significant tumor shrinkage (23). In this study, our results demonstrated that the median PFS of patients with IMDC favorable risk was significantly longer than that of patients in the intermediate-risk group (median 27.1 vs. 17.2 months, respectively). According to the results of a previous study, IMDC favorable-risk patients seem to be the ideal target population for pazopanib treatment, once again indicating the good efficacy of VEGFR targeting agents in patients at low risk (24). An ongoing controversy exists regarding the treatment of patients with IMDC intermediate risk. For patients in the IMDC intermediate- or poor-risk groups, the recommended standard treatment according to the Checkmate-214 trial is the combination of nivolumab plus ipilimumab (13). In our study, PFS was much longer in intermediate-risk patients with 1 risk factor than in those with 2 risk factors (median 25.9 vs. 11.2 months, respectively; P<0.0001). Previous retrospective studies of intermediate-risk patients with mRCC receiving targeted agents also found prolonged survival time in patients with 1 risk factor compared with 2 risk factors (16-18). In addition, the results of our analysis revealed that the response rate of pazopanib was 29% when treating mRCC, and that the response rate was significantly related to IMDC risk stratification. The findings from our study and previous studies suggest that mRCC patients in the IMDC intermediate-risk group can be further stratified into 1 risk factor versus 2 risk factors to improve patient outcomes by more accurately guiding clinical treatment (16-18).
Multicenter, large-scale retrospective studies have demonstrated that the most frequent sites of metastasis are the lung, lymph nodes, bone, liver, adrenal, and brain. Less frequent sites of metastasis (<5%) include the pancreas, pleura, peritoneum, spleen, thyroid, and bowel (25,26). In our analysis, consistent with previous studies (25,26), a total of 61.6% (88/143) patients had mRCC with lung metastasis, of which 30.8% (44/143) had lung metastasis only and the remainder had multiple metastases including lung metastasis. One previous study found that the median survival of mRCC with lung metastasis and bone metastasis was 25.1 vs. 19.4 months, respectively (25). Similarly, our study indicated that patients with lung metastasis only had longer PFS than those with bone metastasis (median 25.9 vs. 21.2 months, respectively). In addition, patients with multiple metastases including lung metastasis had longer PFS than those with multiple metastases without lung metastasis (median 31.0 vs. 11.21 months, respectively).
The major advantage of our study was its use of real-world, multicenter data to provide insight into the effectiveness and tolerability of pazopanib in clinical practice, which can be contrasted with the more selected patient populations entering prospective clinical trials. Another strength was that our study analyzed the survival profiles, metastasis features, and drug safety of patients receiving pazopanib monotherapy based on IMDC risk stratification and risk factors. However, our study had several obvious drawbacks. According to the NCCN recommendations, the initial treatment for IMDC favorable- and intermediate-risk patients should consist of combination therapy of a VEGFR inhibitor and a PD-1/PD-L1 antibody. However, the potential limitations of drug accessibility, affordability, and tolerability of the drug dose have forced some patients to take targeted monotherapy as a first-line treatment. This is an inevitable problem in real-world clinical practice. In addition, due to the retrospective nature of this study and the variations in the extent of adherence across patients, we could not account for all the biases in our study. Toxicity reports from retrospective, unmonitored studies are inevitably less accurate than those from prospective studies. Additionally, missing data may have affected the accuracy of the results, as data on possible subsequent dose changes and the relationship between different doses of pazopanib and their efficacy were unavailable in our database. However, our results were consistent with previous results reported in both real-world studies and clinical trials.
Acknowledgments
Thanks to Novartis staff for their statistical support for this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-312/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-312/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-312/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Fudan University Shanghai Cancer Center (No. 050432-4-1911D, Shanghai, China). All patients participating in this study signed informed consent forms.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol 2016;70:93-105. [Crossref] [PubMed]
- Tanaka N, Mizuno R, Ito K, et al. External Validation of the MSKCC and IMDC Risk Models in Patients Treated with Targeted Therapy as a First-line and Subsequent Second-line Treatment: A Japanese Multi-institutional Study. Eur Urol Focus 2016;2:303-9. [Crossref] [PubMed]
- Hakimi AA, Voss MH, Kuo F, et al. Transcriptomic Profiling of the Tumor Microenvironment Reveals Distinct Subgroups of Clear Cell Renal Cell Cancer: Data from a Randomized Phase III Trial. Cancer Discov 2019;9:510-25. [Crossref] [PubMed]
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794-9. [Crossref] [PubMed]
- Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol 2013;14:141-8. [Crossref] [PubMed]
- Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol 2018;19:405-15. [Crossref] [PubMed]
- Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 2020;21:1563-73. [Crossref] [PubMed]
- Choueiri TK, Larkin J, Oya M, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol 2018;19:451-60. [Crossref] [PubMed]
- Kawakami F, Sircar K, Rodriguez-Canales J, et al. Programmed cell death ligand 1 and tumor-infiltrating lymphocyte status in patients with renal cell carcinoma and sarcomatoid dedifferentiation. Cancer 2017;123:4823-31. [Crossref] [PubMed]
- Fridman WH, Zitvogel L, Sautès-Fridman C, et al. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 2017;14:717-34. [Crossref] [PubMed]
- Motzer RJ, Jonasch E, Boyle S, et al. NCCN Guidelines Insights: Kidney Cancer, Version 1.2021. J Natl Compr Canc Netw 2020;18:1160-70. [Crossref] [PubMed]
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018;378:1277-90. [Crossref] [PubMed]
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380:1116-27. [Crossref] [PubMed]
- Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med 2021;384:1289-300. [Crossref] [PubMed]
- Tamada S, Iguchi T, Yasuda S, et al. The difference in the survival rate of patients with metastatic renal cell carcinoma in the intermediate-risk group of the Memorial Sloan Kettering Cancer Center criteria. Oncotarget 2018;9:27752-9. [Crossref] [PubMed]
- Sella A, Michaelson MD, Matczak E, et al. Heterogeneity of Patients With Intermediate-Prognosis Metastatic Renal Cell Carcinoma Treated With Sunitinib. Clin Genitourin Cancer 2017;15:291-299.e1. [Crossref] [PubMed]
- Iacovelli R, De Giorgi U, Galli L, et al. Is It Possible to Improve Prognostic Classification in Patients Affected by Metastatic Renal Cell Carcinoma With an Intermediate or Poor Prognosis? Clin Genitourin Cancer 2018;16:355-359.e1. [Crossref] [PubMed]
- Chen J, Ye W, Jiang W, et al. Pazopanib in patients with metastatic renal cell carcinoma: a single-center, real-world, retrospective Chinese study. Transl Androl Urol 2021;10:1321-31. [Crossref] [PubMed]
- Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol 2017;35:591-7. Erratum in: J Clin Oncol 2017;35:3736 Erratum in: J Clin Oncol 2018;36:521. [Crossref] [PubMed]
- Escudier B, Motzer RJ, Tannir NM, et al. Efficacy of Nivolumab plus Ipilimumab According to Number of IMDC Risk Factors in CheckMate 214. Eur Urol 2020;77:449-53. [Crossref] [PubMed]
- Procopio G, Bamias A, Schmidinger M, et al. Real-world Effectiveness and Safety of Pazopanib in Patients With Intermediate Prognostic Risk Advanced Renal Cell Carcinoma. Clin Genitourin Cancer 2019;17:e526-33. [Crossref] [PubMed]
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30:706-20. [Crossref] [PubMed]
- McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 2018;24:749-57. Erratum in: Nat Med 2018;24:1941. [Crossref] [PubMed]
- Dudani S, de Velasco G, Wells JC, et al. Evaluation of Clear Cell, Papillary, and Chromophobe Renal Cell Carcinoma Metastasis Sites and Association With Survival. JAMA Netw Open 2021;4:e2021869. [Crossref] [PubMed]
- Karacin C, Bilgetekin I, Basal FB, et al. Prognostic Importance of Metastatic Site in Intermediate-risk Group Metastatic Renal Cell Cancer Treated with Tyrosine Kinase Inhibitors. J Coll Physicians Surg Pak 2020;30:590-4. [Crossref] [PubMed]
(English Language Editor: C. Goulay)



