
Urinary extracellular vesicle microRNA profiling for detection in patients with interstitial cystitis
Interstitial cystitis (IC) is characterized by pelvic pain, pressure, or discomfort related to the urinary bladder, often accompanied by urinary urgency or frequency and relieved by voiding (1). However, diagnosis varies widely depending on the urologist’s experience. The identification of a novel objective biomarker for IC could greatly improve diagnostic consistency and expedite timely intervention where needed. Recently, liquid biopsy using microRNA (miRNA) in biofluids shows promise for early cancer detection (2). miRNAs are small noncoding RNAs consisting of about 20 nucleotides. They can be stably present in extracellular vesicles (EVs), and the profiles of miRNA expression in EVs make them good candidates for improving the diagnosis of a variety of diseases. This paper describes our comprehensive analysis of the urinary EV miRNA expression profile and evaluates its potential as a novel biomarker in IC/BPS patients.
Urinary samples were obtained from 8 Hunner type IC (HIC) patients, 2 bladder pain syndrome (BPS) patients, and 10 non-IC/BPS patients (controls) who were diagnosed with stress urinary incontinence and/or pelvic organ prolapse. The definitions of HIC and BPS followed the recent Japanese guideline (1). Briefly, the definition of IC/BPS is the condition with chronic pelvic pain, pressure or discomfort perceived to be related to the urinary bladder accompanied by other urinary symptoms, such as persistent urge to void or urinary frequency in the absence of confusable diseases (1). Additionally, IC/BPS is divided into HIC and BPS. HIC represents IC/BPS with Hunner lesion, and BPS represents IC/BPS without Hunner lesion (1). All HIC and BPS patients were clinically diagnosed by cystoscopy before urine collection. All patients had a urinary analysis to rule out urinary tract infection. All controls did not have any lower urinary tract symptom. Urine was collected through a urethral catheter and immediately centrifuged at 1,500 rpm for 5 min, and the supernatant was frozen at −80 ℃ pending analysis. Cystoscopy and urine collection were performed at least 7 days apart. We collected urinary EV miRNA using a nanowire-based device (CRAIF Inc.) (3,4). Comprehensive miRNA profiles for all samples were analyzed using a 3D-Gene miRNA Labelling kit and 3D-Gene Human miRNA Oligo Chip (Toray Industries). Leave-one-out cross-validation was used to identify the top 20 miRNAs by accuracy of correctly classifying IC/BPS and controls in receiver operating characteristic (ROC) curve analysis; miRNAs with area under the curve (AUC) values ≥0.85 and cross-validated accuracy ≥0.85 were selected. The cut-off levels were selected using Youden index. All HIC and BPS patients completed the O’Leary-Sant score symptom indexes (OSSI), O’Leary-Sant score problem indexes (OSPI), and visual analog scale (VAS) scores. The correlation between these scores and the level of miRNA expression was analyzed from the Pearson correlation. P<0.05 was regarded as statistically significant. Details of the methods and statistics are described in Appendix 1.
Characteristics of the study population are detailed in Table 1. The cohort of 3 men and 17 women had an average age of 53.3 years (range, 23–73 years). A total of 485 miRNAs passed the quality check criteria and were selected for further study. Principal component analysis (PCA) mapping and heatmap of these miRNAs provided a rough separation of HIC, BPS, and controls (Figure 1A,1B). The volcano plot suggested differences in signal intensity between IC/BPS and controls (Figure S1). miR-375-5p showed an AUC of 0.89 and cross-validated accuracy of 0.90, and miR-6766-5p showed an AUC of 0.86 and cross-validated accuracy of 0.85 (Figure 1C, Table 2). The signal intensity of miR-6766-5p was significantly higher, and of miR-373-5p was significantly lower, in HIC patients than in the control patients (Figure S1B,S1C). In addition, we evaluated diagnostic performance of miR-375-5p and miR-6766-5p between HIC patients and controls, which also showed high accuracy, respectively (miR-375-5p: AUC, 0.86; sensitivity, 0.88; specificity, 0.90; and miR-6766-5p: AUC, 0.86; sensitivity, 0.88; specificity, 0.80) (Figure S2). Although some trends were noted for miR-373-5p, no significant association between signal intensity and quality of life (QOL) scores was detected for either miRNA (Figure S3). As the body mass index (BMI) was significantly higher in controls, we also evaluated the association between the two miRNAs and BMI. There was no significant association between them (Figure S4).
Table 1
| Characteristics | HIC (n=8) | BPS (n=2) | Control (n=10) | P value (HIC/BPS vs. control) |
|---|---|---|---|---|
| Female | 6 (75.0%) | 1 (50%) | 10 (100%) | 0.06 |
| Age, years | 59.9 (10.7) | 49 (5.7) | 48.9 (18.4) | 0.325 |
| BMI, kg/m2 | 22.3 (2.2) | 19.2 (1.1) | 25.7 (3.9) | 0.041 |
| OSPI | 11.5 (4.0) | 10 (0.0) | NA | NA |
| OSSI | 13.3 (3.8) | 8.5 (0.7) | NA | NA |
| VAS score | 7.7 (3.1) | 7 (0.0) | NA | NA |
Values are n (%) or mean (SD). HIC, Hunner type interstitial cystitis; BPS, bladder pain syndrome; BMI, body mass index; OSPI, O’Leary-Sant score problem indexes; NA, not available; OSSI, O’Leary-Sant score symptom indexes; VAS, visual analog scale.
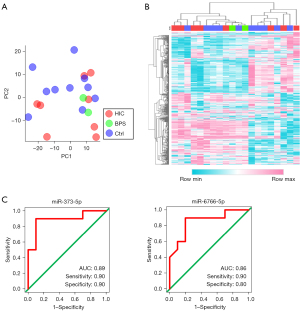
Table 2
| Rank | miRNA | AUC | Cross-validated accuracy |
|---|---|---|---|
| 1 | miR-373-5p | 0.89 | 0.90 |
| 2 | miR-6766-5p | 0.86 | 0.85 |
| 2 | miR-6813-5p | 0.79 | 0.85 |
| 3 | miR-1181-3p | 0.82 | 0.80 |
| 3 | miR-4638-5p | 0.85 | 0.80 |
| 3 | miR-4648 | 0.80 | 0.80 |
| 3 | miR-4730 | 0.77 | 0.80 |
| 3 | miR-4731-5p | 0.84 | 0.80 |
| 3 | miR-6090 | 0.81 | 0.80 |
| 3 | miR-6778-5p | 0.80 | 0.80 |
| 3 | miR-6872-3p | 0.79 | 0.80 |
| 4 | miR-204-3p | 0.76 | 0.75 |
| 4 | miR-2392 | 0.73 | 0.75 |
| 4 | miR-3188 | 0.74 | 0.75 |
| 4 | miR-371a-5p | 0.80 | 0.75 |
| 4 | miR-375-5p | 0.70 | 0.75 |
| 4 | miR-4447 | 0.83 | 0.75 |
| 4 | miR-4454 | 0.73 | 0.75 |
| 4 | miR-4476 | 0.77 | 0.75 |
| 4 | miR-4675 | 0.77 | 0.75 |
IC, interstitial cystitis; AUC, area under the curve.
Our study identified two miRNAs (miR-373-5p and miR-6766-5p) in EVs that will be useful in diagnosing IC/BPS patients. Previously, Liu et al. (5) evaluated the diagnostic value of miR-19a-3p and long noncoding RNA (MEG3) in urinary EVs, but they focused on previously studied RNA (6). To the best of our knowledge, this is the first study that has comprehensively investigated the diagnostic value of urinary EV miRNAs in IC/BPS patients. One strength of this study is that we examined the expression profiles of 2,632 miRNAs, constituting all the human miRNA identified to date according to miRbase release 22. One weakness is the omission of functional analysis in patients with IC/BPS of the two miRNAs (miR-373-5p and miR-6766-5p), which we selected as candidate miRNAs for diagnosis. The sample size was small, and the samples were collected in a single institution. Additionally, only the difference of the miRNA profiles between IC/BPS and controls were evaluated. In clinical, the differences of the profiles between IC/BPS and hyper sensitive bladder might be more useful. Further investigation of clinical applications will thus be required. However, our pilot study showed that urinary EV miRNAs are potentially useful for diagnosing IC. This point may prove beneficial to urologists in daily clinical practice in the near future.
Acknowledgments
We thank Kodai Minoura and Hiroki Yamaguchi (CRAIF Inc.) for their skilled assistance with statistical analysis.
Funding: This work was supported by Kanzawa Medical Research Foundation, Takeda Science Foundation, The Japanese Urological Association, and a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science (grant number 21K16758).
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-240/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-240/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol for this study was approved by The Jikei University Institutional Review Board [No. 32-284(10366)]. Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Homma Y, Akiyama Y, Tomoe H, et al. Clinical guidelines for interstitial cystitis/bladder pain syndrome. Int J Urol 2020;27:578-89. [Crossref] [PubMed]
- Urabe F, Kosaka N, Yoshioka Y, et al. The small vesicular culprits: the investigation of extracellular vesicles as new targets for cancer treatment. Clin Transl Med 2017;6:45. [Crossref] [PubMed]
- Yasui T, Yanagida T, Ito S, et al. Unveiling massive numbers of cancer-related urinary-microRNA candidates via nanowires. Sci Adv 2017;3:e1701133. [Crossref] [PubMed]
- Kitano Y, Aoki K, Ohka F, et al. Urinary MicroRNA-Based Diagnostic Model for Central Nervous System Tumors Using Nanowire Scaffolds. ACS Appl Mater Interfaces 2021;13:17316-29. [Crossref] [PubMed]
- Liu F, Chen Y, Liu R, et al. Long noncoding RNA (MEG3) in urinal exosomes functions as a biomarker for the diagnosis of Hunner-type interstitial cystitis (HIC). J Cell Biochem 2020;121:1227-37. [Crossref] [PubMed]
- Arai T, Fuse M, Goto Y, et al. Molecular pathogenesis of interstitial cystitis based on microRNA expression signature: miR-320 family-regulated molecular pathways and targets. J Hum Genet 2018;63:543-54. [Crossref] [PubMed]


