
MiRNA-148a inhibits cell growth and drug resistance by regulating WNT10a expression in renal cell carcinoma
Introduction
Renal cell carcinoma (RCC) affects urinary system which is a common malignant tumor, and it accounts for the majority of urogenital tumors in China. More than 350,000 patients are newly diagnosed with RCC every year (1-3). Although RCC can occur at any age, its incidence increases with age, with a high incidence among those aged 50–70 years (4,5). The cause of RCC is a pathological process of complex, multi-step, and multi-factor, which is a result of the interaction between intrinsic genetic factors and external environmental factors (6,7). Clinically, RCC is treated with radical nephrectomy. However, postoperative recurrence and metastasis rates of RCC are high, and 5-year overall survival rates are about 20% (8). Currently, RCC, which shows little response to chemoradiotherapy, is undergoing rapid development with the application of targeted therapy (9). Arsenic trioxide (As2O3) is a Food and Drug Authority (FDA)-approved drug used to treat acute promyelocytic leukemia (APL). Approximately 65.6–84.4% of patients treated with As2O3 achieve clinical remission, and over 28.2% survive at least 10 years after treatment with As2O3(10). It has been reported that As2O3 induces apoptosis in RCC cells, however only high concentrations of As2O3 can achieve this effect, which may be due to the resistance of RCC cells to As2O3 (11). The RCC mortality rate is the highest among urinary tumors, which seriously endangers the life and health of those affected. Therefore, understanding the molecular genetic mechanism of RCC and exploring the molecular mechanism of tumor resistance to As2O3 and treatment of RCC are a current research hotspot (12,13).
MicroRNA (miRNA) is a class of endogenous non-coding RNA consisting of 22 nucleotides that bind completely, or in the instance of not reaching 3' untranslated region (3'-UTR) of the target gene, the messenger RNA (mRNA) is degraded or its protein expression is diminished, exerting an oncogenic or tumor suppressor genes effect (14,15). Among them, miR-148a is related to the occurrence and development of the tumors, and exerts an anti-cancer effect in gastric cancer and laryngeal squamous cell carcinoma (16,17). Previous study has reported that epigenetic regulation of miR-148a by As2O3 is an important mechanism of drug resistance (18). However, there are few reports about the role in RCC. In our study, we explored whether miR-148a could play a role of tumor resistance to As2O3 in RCC and investigated the specific mechanism of its anti-cancer effect, thus providing new experimental clues for the treatment of R CC. Previous studies targeting miRNAs in RCC have focused on diagnosis, prognosis and drug sensitivity (19). The expression of miR-148a was found to be closely related to RCC by TCGA database analysis (20). However, the mechanism of As2O3 resistance and its relationship with miR-148a have not been reported. Further study of the molecular biological role of miR-148a will help to discover biomarkers of As2O3 resistance in RCC patients.
Recently, many studies have shown that Wnt family member 10A (WNT10a) has oncogene properties (21). A study showed that WNT10a was highly overexpressed in human cholangiocarcinoma, and the Wnt pathway was activated during the occurrence of bile duct carcinoma. Previous study reported that WNT10a is high expression in RCC tissues; tumorigenesis can be induced by WNT10a-meditated activation of the wnt/β-catenin signaling pathway (22). Overexpression of WNT10a induced higher resistance of the renal cancer cell line 786-O to chemotherapy, and WNT10a small interfering RNA (siRNA) knockdown reduced the resistance to chemotherapy in renal cancer cell line Caki-1 (23). However, whether WNT10a participates in RCC cell proliferation, induction of apoptosis, and drug resistance mechanism of As2O3 in RCC remains unclear. It has recently been discovered that miR-148a is a novel therapeutic target for human tumors and has been found to regulate WNT10a signaling in a variety of tumor cells. Based on the above series of results, to explore whether miR-148a and WNT10a participate in RCC, our study elucidated the roles of miR-148a and WNT10a in the drug resistance of RCC, and thus may bring new ideas to the treatment of RCC. We present the following article in accordance with the MDAR reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-464/rc).
Methods
Patients and RCC samples
We collected 42 pairs of tumor and paracancerous samples from patients undergoing radical surgery for RCC. Prior to surgery, none of the RCC patients received chemotherapy or radiotherapy. In accordance with the International Cancer against Cancer (UICC) RCC staging standard, pathological classification and staging criteria for RCC were determined. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Review Board of Harbin Medical University Cancer Hospital (No. 2019-185) and informed consent was taken from all the patients.
Cell lines and reagents
We purchased 5 cell lines (769P, ACHN, Caki-2, 786-O, and A-498) as well as human renal proximal tubule epithelial cells (TH1) from the American Type Culture Collection (ATCC; Manassas, VA, USA). Fetal bovine serum (FBS) was obtained from Gibco (Rockville, MD, USA). Dulbecco’s modified Eagle medium (DMEM) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). An incubator with 5% CO2 at 37 ℃ was used to culture the cells in DMEM containing 10% FBS.
Transfection
Shanghai Jima Company provided the miR-NC and miR-148a mimics for this study. Transfection was performed using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA). Cell functional and real-time polymerase chain reaction (RT-PCR) analysis was performed after cultured for 48 hours.
Cell Counting Kit-8 assay
After transfection for 48 hours, 96-well plates were filled with 2,000 cells per well. We added Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) reagent to the cells which were cultured for 24 hours, 48 hours, 72 hours, and 96 hours. After incubation for 2 hours, the optical density (OD) value was detected at 490 nm in a microplate reader.
Transwell assay
After transfection for 48 hours, the cells were adjusted to 5×105 cells/mL by resuspending them in medium without FBS. The upper chamber was infused with 200 µL of a medium containing 1×105 cells, and the lower chamber with 700 uL of a medium containing 20% FBS. For each cell line, a specific period of growth was determined based on their migration abilities. Transwell chambers were clipped, washed with phosphate-buffered saline (PBS) for 3 times. We used a cotton swab to clean the top surface of the stained chamber with water after it had been stained for 20 minutes with 0.2% crystal violet. A random selection of 5 fields of view were taken under a microscope and the perforated cells stained on the outer surface of the basement membrane were observed.
Cell wound healing assay
After transfection for 48 hours, the cells were adjusted to 5×105 cells/mL by resuspending them in medium without FBS. Cells were plated at varying densities based on the size of the cells (50,000 cells per well was the majority), 90% or more of the cells were confluent the following day. As soon as the cells had been rinsed with PBS for 3 times, the medium was added with 1% FBS. In the pre-experiment, the healing ability of the cells is determined according to migration area; the differences in healing ability were evaluated based on migration area for the scratch test.
qRT-PCR
We used TRIzol (Invitrogen, USA) to lyse the cells. Then total RNA was extracted after the cells were treated accordingly. After initially removing genomic DNA from RNA, the RNA was purified using DNase I. The Prime Script Reverse Transcription Kit (TaKaRa, Otsu, Shiga, Japan) instructions were followed for reverse transcription of RNA, and RT-PCR was performed according to SYBR® Premix Ex TaqTM (TaKaRa, Japan) kit using the StepOne Plus Real-time PCR System [Applied Biosystems (AB), Foster City, CA, USA]. For each sample, 3 replicate wells were performed and repeated the assay twice. We analyzed the data using a Bio-Rad PCR instrument and software iQ5 2.0 (Bio-Rad Laboratories, Hercules, CA, USA). As internal parameters, we used the β-actin and U6 genes, and we calculated gene expression using the 2-ΔΔCt method.
Dual-luciferase reporting assay
According to the instructions, the luciferase system was used to prepare the logarithmic growth RCC cell line. By using a luminometer, relative fluorescence values were measured and compared. It was found that when a specific miRNA could complementarily bind to a target gene sequence in the system, it was detected. Luciferase was not expressed, so the final relative fluorescence value measured was lower than the sequence.
Flow cytometry
Apoptosis was determined using flow cytometry. Briefly, apoptosis of cells was assessed by staining with AnnexinV and PE/7-AAD using the Apoptosis Detection Kit (Cat. No. C1062S, Beyotime Biotech., Shanghai, China). Then, 100 µL of the 1×105 cells were stained with AnnexinV-PE (5 µL) and 7-AAD (5 µL) for 20 minutes at room temperature. Cells in each well were incubated with IX binding buffer (400 µL). We analyzed the cell apoptosis using a FACScan flow cytometer (BD, Mountain View, CA, USA).
Statistical analysis
The statistical analysis was conducted using the software SPSS 22.0 (IBM Corp., Armonk, NY, USA). A univariate analysis was performed using the χ2-test and Fisher’s test was used to determine the exact probability. We used Cox regression analysis for multivariate analysis and Kaplan-Meier for analyzing patient survival. Comparison of the intergroup curves was performed using the log-rank test. Statistical significance was determined by P<0.05 when data were expressed as mean ± standard deviation.
Results
Low-expression of miR-148a in RCC tissues and cell lines
Figure 1A showed the PCR results showed that the level of miR-148a was noticeably lower in RCC patients’ tumors than in adjacent tissues. Moreover, miR-148a was remarkably downregulated, and there was a significant difference, especially in the Caki-1 and 786-O cell lines, which were selected for the following (Figure 1B). Table 1 showed that low miR-148a expression was positively associated with distant metastasis in RCC (Figure 1C), but not with age, gender, stage of the disease, or lymph node metastasis. Furthermore, Figure 1D showed that the Kaplan–Meier survival curve results revealed that low miR-148a expression was remarkably associated with poor prognosis for RCC patients (P<0.05). Our results found that miR-148a serve as a new biological indicator of malignant progression of RCC.
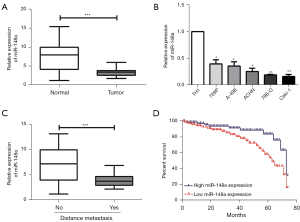
Table 1
| Parameters | Number of cases | miRNA-148a expression | P value | |
|---|---|---|---|---|
| High | Low | |||
| Age (years) | 0.850 | |||
| <60 | 16 | 10 | 6 | |
| ≥60 | 26 | 17 | 9 | |
| Gender | 0.461 | |||
| Female | 22 | 13 | 9 | |
| Male | 20 | 14 | 6 | |
| T stage | 0.628 | |||
| T1-T2 | 26 | 15 | 9 | |
| T3-T4 | 16 | 10 | 6 | |
| Lymph node metastasis | 0.172 | |||
| Yes | 14 | 7 | 7 | |
| No | 28 | 20 | 8 | |
| Distance metastasis | 0.024 | |||
| Yes | 11 | 4 | 7 | |
| No | 31 | 23 | 8 | |
Over-expression of miR-148a inhibited cell proliferation and metastasis
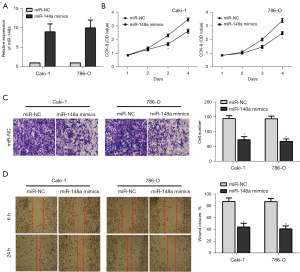
The miR-148a mimics model was performed and verified by RT-PCR for transfection efficiency (Figure 2A). CCK-8, cell scratch assays, and transwell migration were conducted in 786-O and Caki-1cell lines. Figure 2B showed that the miR-148a mimics group had significantly lower proliferation compared to the miR-NC group. Subsequently, the transwell migration suggested that the number of perforated RCC tumor cells in the miR-148a mimics group was significantly reduced compared to the miR-NC group (Figure 2C). In addition, according to the scratch assays performed on RCC cells using miR-148a mimics, the ability of these cells to crawl was also considerably decreased compared to miR-NC cells (Figure 2D). These results indicated that the miR-148a over-expression inhibited cell proliferation and metastasis.
WNT10a was highly expressed in RCC tissues and cell lines
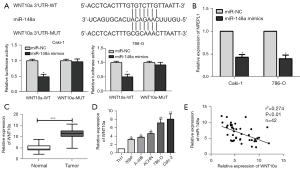
By bioinformatics prediction, we found that WNT10a might be a possible target of miR-148a. We further verify the targeting of miR-148a to WNT10a, miR-148a, and WNT10a were co-transfected into 786-O and Caki-1cell lines for luciferase reporter gene experiments. Our results showed that miR-148a is targeted by WNT10a via this binding site (Figure 3A). Furthermore, Figure 3B showed that WNT10a expression levels were remarkably reduced after overexpression of miR-148a in the 786-O and Caki-1. The above series of experiments showed that miR-148a can affect the malignant progression of RCC by regulating WNT10a. Subsequently, we performed RT-PCR to detect the WNT10a expression in cell lines and tissues, and the results showed that the WNT10a expression levels were significantly increased compared to these of the adjacent tissues (Figure 3C). In addition, WNT10a was remarkably highly expressed in RCC cells compared to SV-HUC-1, and the difference was also statistically significant (Figure 3D). Therefore, the miR-148a and WNT10a expressions were remarkably negatively correlated (Figure 3E).
MiR-148a exactly inhibited WNT10a gene expression in RCC
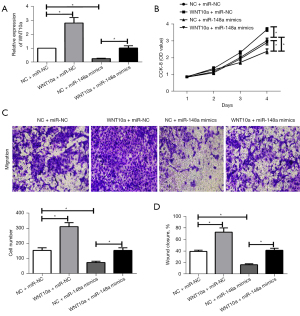
We further explore whether miR-148a inhibit malignant progression by WNT10a, WNT10a was overexpressed in Caki-1 cell lines transfected with miR-148a and miR-NC mimics to investigate their role in RCC by RT-PCR. We examined the transfection efficiency of WNT10a (Figure 4A). Subsequently, the CCK-8 assay, transwell migration assay, and cell scratch assay demonstrated that WNT10a overexpression could offset the miR-148a mimics on RCC cells proliferation and metastasis (Figure 4B-4D). In sum, these results demonstrated that miR-148a regulated the RCC cells’ proliferation and metastasis via inhibition of WNT10a gene expression.
WNT10a reversed the apoptosis of MiR-148a after As2O3 treatment of Caki-1 cells
To investigate the effect of miR-148a on RCC cell proliferation, we assessed the ability of miR-148a to sensitize Caki-1 cells treated with As2O3 using flow cytometry. We detected relative apoptosis after As2O3 was treated with or without miR-148a mimics for 48 hours. The Caki-1 cells were treated with As2O3 at a concentration of 0.5 µM for 48 hours. After transfection of miR-148a mimics into the Caki-1 cell line, apoptosis was significantly enhanced after combined treatment compared with As2O3 treatment alone. In contrast, apoptosis was significantly reduced after transfection of WNT10a in the Caki-1 cell line, and significantly reduced after combined treatment (Figure 5).

Discussion
Around 90% of kidney cancers are classifies as RCC, which is a very invasive and chemoresistant disease and responds poorly to radiotherapy. The incidence of RCC has increased worldwide at an annual rate of 1.5–5.9% and accounts for about 3% of all human cancers. Almost 25–30% patients with RCC have metastatic disease at the time of diagnosis and survival outcomes for these patients are suboptimal, and the 5-year progression-free survival rate of only 30% (1-4). Therefore, finding new effective treatment methods for RCC provides new theoretical basis for treatment of tumors, and has important clinical significance for improving the survival rate and cure rate of cancer patients (9-12).
The miRNA is a class of endogenous single-stranded small RNA that regulate life activities. Although miRNAs do not encode proteins themselves, they can regulate the target gene expression by binding completely or incompletely to the 3'-UTR, resulting in a target gene mRNA degradation or inhibition of its protein expression (14,15). A number of studies have found that the dysregulation of miRNA expression is an important factor in tumorigenesis and progression, suggesting that the mechanisms by which miRNAs work are important for developing new strategies for cancer prevention and treatment. Numerous studies have investigated the regulatory mechanisms of miRNAs expression in tumorigenesis and development (24,25). Since 2007, people began to characterize the transcriptome of renal cell carcinoma, and often followed up, using more centralized technology, focusing on using the characteristics of renal cell carcinoma as a diagnostic and prognostic tool. Several studies have paired maladjusted miRNAs with complementary specific genes based on the 3'-UTR sequence of RCC sample genes with mRNA targets that predict seeds (26). These bioinformatics analyses have led to a large number of hypothetical miRNA mRNA networks. Each target gene may be affected by multiple miRNAs and miRNA families. Many miRNAs that predict the interaction with tumor pathway related genes are significantly up-regulated or down regulated in other tumor types, so they can be regarded as oncogenes or tumor suppressors in RCC, respectively (27).
Many studies have indicated that miR-148a is a tumor suppressor (16-18). A study concluded that miR-148a inhibits the malignant progression of colorectal cancer by mediating programmed death ligand-1 (PD-L1) expression (28). Another study (29) showed that miR-148a inhibits cell invasion and proliferation of esophageal cancer by targeting Dnmt1. Therefore, we decided to explore whether miR-148a also exhibits this imbalance in RCC. In this experiment, the miR-148a expression in RCC tissues and the corresponding adjacent tissues was detected using RT-PCR. Our results showed that miR-148a level in RCC was remarkably lower than that of the corresponding cancers, suggesting that miR-148a also had this dysregulated expression in RCC, and that miR-148a may play an important inhibitory role in the development of RCC. Subsequently, in order to further explore the effect of miR-148a on the biological function of RCC, the miR-148a mimics model was constructed. The results of CCK-8, transwell migration assay, and cell scratch assay showed that miR-148a can inhibit the cell proliferation and metastasis of RCC.
A traditional remedy in Chinese medicine, As2O3 (ATO) inhibits growth and promotes apoptosis in many different cancer cell types. There have been some reports on the effects of As2O3 on RCC. For instance, a study showed that As2O3 inhibited the A498 renal cancer cells growth through inducing apoptosis or cell cycle arrest, As2O3 also decreased CDK2, CDK6, and cyclin D1 levels after treatment on A498 cells. It increases the sensitivity of 786-O cells to radiotherapy (30). It has been found that galectin-3 (Gal-3) inhibition sensitized human RCC cells to As2O3 treatment, suggesting the potential synergetic use of As2O3 and Gal-3 inhibition for RCC treatment (31). These studies indicate that As2O3 may indeed inhibit the RCC cells proliferation and promote apoptosis. Previous study reported that epigenetic regulation of miR-148a by As2O3 is an important mechanism of drug resistance (18). Therefore, we speculate that miR-148a has the same function in RCC.
Numerous studies have shown that miRNAs affect the development of tumors by regulating their downstream target genes (32). Therefore, in this experiment, we used bioinformatics software to predict the downstream target genes of miR-148a, and found that miR-148a had a complementary binding site at the 3'-UTR of WNT10a mRNA, suggesting that miR-148a may target the regulation of WNT10a expression. To further confirm that WNT10a was a target gene for miR-148a, we used a dual-luciferase gene reporter system for further study. This study found that miR-148a can inhibit the WNT10a expression in RCC cells. The WNT10a expression decreased with the miR-148a expression in a dose-dependent manner. In addition, WNT10a promotes the proliferation and metastasis, and miR-148a affects the WNT10a expression in RCC cells. MiR-148a negatively regulated WNT10a, suggesting that WNT10a may be a target gene for miR-148a. To further explore the effect and interaction of miR-148a and WNT10a on the progression and development of RCC, recovery experiments were conducted and verified that the overexpression of WNT10a can offset the effect of miR-148a mimics on the proliferation and metastasis of RCC cells. To investigate the effect of miR-148a on RCC cell proliferation, we found that As2O3 could be treated with or without miR-148a mimics to promote apoptosis in renal cancer cells, but WNT10a reversed this trend. Our findings suggested that there may be a feedback regulation loop, in which WNT10a can reverse the biological role of miR-148a in RCC cells, thereby jointly regulating the malignant progression and drug resistance of RCC.
Conclusions
In summary, the study revealed that miR-148a is associated with distant metastases and leads to poor prognosis in RCC patients. Moreover, miR-148a inhibits the malignant progression and increase the sensitivity of RCC cells to As2O3 by regulating WNT10a.
Acknowledgments
Funding: The work was supported by Special Fund Youth Reserve Talent Project for Harbin Science and Technology Innovation Talent Research (No. 2016RAQXJ150).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-464/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-464/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-464/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Review Board of Harbin Medical University Cancer Hospital (No. 2019-185) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Palacios DA, Zabor EC, Munoz-Lopez C, et al. Does Reduced Renal Function Predispose to Cancer-specific Mortality from Renal Cell Carcinoma? Eur Urol 2021;79:774-80. [Crossref] [PubMed]
- Jian Y, Yang K, Sun X, et al. Current Advance of Immune Evasion Mechanisms and Emerging Immunotherapies in Renal Cell Carcinoma. Front Immunol 2021;12:639636. [Crossref] [PubMed]
- Rysz J, Franczyk B, Ławiński J, et al. Characteristics of Clear Cell Papillary Renal Cell Carcinoma (ccpRCC). Int J Mol Sci 2021;23:151. [Crossref] [PubMed]
- Shen H, Liu J, Liu W, et al. Conditional survival of metastatic clear cell renal cell carcinoma: How prognosis evolves after cytoreductive surgery of primary tumor. Cancer Med 2021;10:7492-502. [Crossref] [PubMed]
- Rizzo A, Mollica V, Dall'Olio FG, et al. Quality of life assessment in renal cell carcinoma Phase II and III clinical trials published between 2010 and 2020: a systematic review. Future Oncol 2021;17:2671-81. [Crossref] [PubMed]
- Panian J, Lin X, Simantov R, et al. The Impact of Age and Gender on Outcomes of Patients With Advanced Renal Cell Carcinoma Treated With Targeted Therapy. Clin Genitourin Cancer 2020;18:e598-609. [Crossref] [PubMed]
- Chen X, Tu J, Ma L, et al. Analysis of Ferroptosis-Related LncRNAs Signatures Associated with Tumor Immune Infiltration and Experimental Validation in Clear Cell Renal Cell Carcinoma. Int J Gen Med 2022;15:3215-35. [Crossref] [PubMed]
- Bruckschen F, Gerharz CD, Sagir A. Renal cell carcinoma with unusual metachronous metastasis up to 22 years after nephrectomy: two case reports. J Med Case Rep 2021;15:490. [Crossref] [PubMed]
- Hicks BM, Chun DS, Peacock Hinton S, et al. Patterns of first-line targeted therapy utilization and adherence among older adults diagnosed with metastatic renal cell carcinoma. J Geriatr Oncol 2022;13:325-33. [Crossref] [PubMed]
- Seong NJ, Yoon CJ, Kang SG, et al. Effects of Arsenic Trioxide on Radiofrequency Ablation of VX2 Liver Tumor: Intraarterial versus Intravenous Administration. Korean J Radiol 2012;13:195-201. [Crossref] [PubMed]
- Xu Y, Gu X, Gong M, et al. Galectin-3 inhibition sensitizes human renal cell carcinoma cells to arsenic trioxide treatment. Cancer Biol Ther 2013;14:897-906. [Crossref] [PubMed]
- Wang Z, Wang J, Zhu Y, et al. Cause-Specific Mortality Among Survivors From T1N0M0 Renal Cell Carcinoma: A Registry-Based Cohort Study. Front Oncol 2021;11:604724. [Crossref] [PubMed]
- Panaiyadiyan S, Quadri JA, Nayak B, et al. Association of heavy metals and trace elements in renal cell carcinoma: A case-controlled study. Urol Oncol 2022;40:111.e11-8. [Crossref] [PubMed]
- Moradi S, Kamal A, Aboulkheyr Es H, et al. Pan-cancer analysis of microRNA expression profiles highlights microRNAs enriched in normal body cells as effective suppressors of multiple tumor types: A study based on TCGA database. PLoS One 2022;17:e0267291. [Crossref] [PubMed]
- Solé C, Lawrie CH. MicroRNAs in Metastasis and the Tumour Microenvironment. Int J Mol Sci 2021;22:4859. [Crossref] [PubMed]
- Zhou H, He Y, Li L, et al. Identification novel prognostic signatures for Head and Neck Squamous Cell Carcinoma based on ceRNA network construction and immune infiltration analysis. Int J Med Sci 2021;18:1297-311. [Crossref] [PubMed]
- Song M, Liu J, Zheng X, et al. MiR-148a-3p targets CEMIP to suppress the genesis of gastric cancer cells. Biochem Biophys Res Commun 2021;575:42-9. [Crossref] [PubMed]
- Wang Y, Jiang F, Jiao K, et al. De-methylation of miR-148a by arsenic trioxide enhances sensitivity to chemotherapy via inhibiting the NF-κB pathway and CSC like properties. Exp Cell Res. 2020;386:111739. [Crossref] [PubMed]
- Peng F, Fan H, Li S, et al. MicroRNAs in Epithelial-Mesenchymal Transition Process of Cancer: Potential Targets for Chemotherapy. International Journal of Molecular Sciences 2021;22:7526. [Crossref] [PubMed]
- Liu C, Gong X, Zhang S, et al. Comprehensive molecular characterization of clear cell renal cell carcinoma with caval tumour thrombus. European Urology Supplements 2019;18:e2100. [Crossref]
- Li J, Zhang Z, Wang L, et al. The oncogenic role of Wnt10a in colorectal cancer through activation of canonical Wnt/β-catenin signaling. Oncol Lett 2019;17:3657-64. [Crossref] [PubMed]
- Piotrowska Ż, Niezgoda M, Mlynarczyk G, et al. Comparative Assessment of the WNT/β-Catenin Pathway, CacyBP/SIP, and the Immunoproteasome Subunit LMP7 in Various Histological Types of Renal Cell Carcinoma. Front Oncol. 2020;10:566637. [Crossref] [PubMed]
- Hsu RJ, Ho JY, Cha TL, et al. WNT10A plays an oncogenic role in renal cell carcinoma by activating WNT/β-catenin pathway. PLoS One 2012;7:e47649. [Crossref] [PubMed]
- Mao Q, Zhuang Q, Shen J, et al. MiRNA-124 regulates the sensitivity of renal cancer cells to cisplatin-induced necroptosis by targeting the CAPN4-CNOT3 axis. Transl Androl Urol 2021;10:3669-83. [Crossref] [PubMed]
- Braga EA, Fridman MV, Filippova EA, et al. LncRNAs in the Regulation of Genes and Signaling Pathways through miRNA-Mediated and Other Mechanisms in Clear Cell Renal Cell Carcinoma. Int J Mol Sci 2021;22:11193. [Crossref] [PubMed]
- Huang Y, Dai Y, Yang J, et al. Microarray analysis of microRNA expression in renal clear cell carcinoma. Eur J Surg Oncol 2009;35:1119-23. [Crossref] [PubMed]
- Valera V A, Walter B A, Marston L W, et al. Regulatory Effects of microRNA-92 (miR-92) on VHL Gene Expression and the Hypoxic Activation of miR-210 in Clear Cell Renal Cell Carcinoma. Journal of Cancer 2011;2:515-26. [Crossref] [PubMed]
- Ashizawa M, Okayama H, Ishigame T, et al. miRNA-148a-3p Regulates Immunosuppression in DNA Mismatch Repair-Deficient Colorectal Cancer by Targeting PD-L1. Mol Cancer Res 2019;17:1403-13. [Crossref] [PubMed]
- Wang Y, Hu Y, Guo J, et al. miR-148a-3p Suppresses the Proliferation and Invasion of Esophageal Cancer by Targeting DNMT1. Genet Test Mol Biomarkers 2019;23:98-104. [Crossref] [PubMed]
- Hyun Park W, Hee Cho Y, Won Jung C, et al. Arsenic trioxide inhibits the growth of A498 renal cell carcinoma cells via cell cycle arrest or apoptosis. Biochem Biophys Res Commun 2003;300:230-5. [Crossref] [PubMed]
- Su Y, Wang X, Xu W, et al. Arsenic trioxide increases the sensitivity of 786-0 renal carcinoma cells to radiotherapy. Cancer Invest 2012;30:114-8. [Crossref] [PubMed]
- Xu Q, Krause M, Samoylenko A, et al. Wnt Signaling in Renal Cell Carcinoma. Cancers (Basel) 2016;8:57. [Crossref] [PubMed]
(English Language Editor: J. Jones)


