
Ganoderma lucidum polysaccharide ameliorated diabetes mellitus-induced erectile dysfunction in rats by regulating fibrosis and the NOS/ERK/JNK pathway
IntroductionOther Section
Erectile dysfunction (ED) refers to the persistent or repeated inability to develop or maintain sufficient penile erection for successful sexual intercourse (1). ED has severe implications on the quality of life of patients as well as their partners (2). Diabetes mellitus (DM) is a kind of metabolic disease marked by hyperglycemia (3). Research has revealed that the incidence of ED in DM patients is about 1.9–4 times greater than that of patients with normal blood glucose; it is also estimated that approximately three-quarters of ED patients have concomitant DM (4). At present, oral phosphodiesterase (PDE) type 5 inhibitors are adopted as the first-line drugs for treating ED. However, an increasing number of studies have demonstrated that these drugs are ineffective in some patients with serious diabetes mellitus-induced erectile dysfunction (DMED). Also, these drugs sometimes have various adverse effects during use (5). Thus, there is an urgent need to identify and develop a safe and effective therapy for DMED.
Fibrosis plays a key role in ED deterioration, which decreases the elasticity and compliance of the penis (6). In addition, ED is strongly correlated to the NOS of the corpus cavernosum (CC). The parasympathetic nerve, non-adrenergic and non-cholinergic nerve endings, and vascular endothelial cells release nitric oxide (NO) under the action of NOS during sexual stimulation (7). Subsequently, NO triggers the activation of guanylate cyclase to augment the generation of cyclic guanosine monophosphate (cGMP), which decreases the concentration of intracellular calcium, facilitates the relaxation of smooth muscle in the CC, and enhances the influx of blood to the penis, resulting in an erection (8). Furthermore, ERK/JNK is always activated during the development of DM (9). Therefore, the modulation of fibrosis, NOS, and the ERK/JNK pathway in the CC are generally accepted as key interventions for treating DMED.
Ganoderma has been one of the most popular remedies in Eastern medicine for centuries, and Ganoderma lucidum polysaccharide (GLP) is the main active ingredient of Ganoderma (10). GLP is frequently prescribed to alleviate refractory myopathy (11), physical frailty (12) and so on. Studies conducted over the last two decades have confirmed that GLP is a potential agent to treat DM (13). Furthermore, reports have also found that GLP displays a potential effect on the regulation of fibrosis (14), NOS (15), and the ERK/JNK pathway (16) in the CC. However, the specific function and detailed mechanism of GLP in DMED are poorly understood.
Hence, in this study, we applied GLP treatment in DMED rats to investigate its impact on fibrosis, NOS, and the ERK/JNK pathway in the CC. We aimed to explore the effect and mechanism of GLP on ameliorating the ED of DMED rats, thereby providing a novel strategy for treating DMED. Herein, for the first time, we disclosed the effect and mechanism of agents on DMED by numerous approaches to make the results more accurate [for example, the apoptosis of the penile tissues was evaluated by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining, immunohistochemistry as well as mitochondrial membrane potential (MMP) detection]. We present the following article in accordance with the ARRIVE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-428/rc).
MethodsOther Section
Animals
Male 12-week-old Sprague-Dawley (SD) rats weighing 250–300 g were supplied by Beijing Vital River Laboratory Animal Technology Co., Ltd. (Animal License No. SCXK Jing 2016-0011) and kept in SPF conditions. A protocol was prepared before the study without registration. All animal experiments were performed with the approval of the Animal Experimentation Ethics Committee of Zhejiang Eyong Pharmaceutical Research and Development Center (Approval No. ZJEY-20211008-01), and the experiments were conducted according to the institutional guidelines for the care and use of animals. All possible efforts were exerted to reduce animal suffering/pain.
Construction and screening of the DMED rat model
Construction and screening of the DMED rat model were performed according to previous research (17). Briefly, after 1 week of acclimatization, the rats were prohibited from food for 16 h and randomized into a normal control (NC) group and DMED group according to the number random table method. Subsequently, all rats [except for the NC group (n=6)] were intraperitoneally treated with streptozotocin (60 mg/kg in 10 mmol/L citrate buffer; BBI Life Sciences, Shanghai, China; GB24BA0501) to induce DM, while rats in the NC group received the same volume of citrate buffer (10 mmol/L). After 7 days of streptozotocin injection, the rats were fasted for 8 h over 3 consecutive days, and then tested the level of fasting blood glucose. Only rats who had a fasting blood glucose level greater than 16.7 mmol/L (over at least two tests) and displayed the symptoms of polyphagia, polydipsia, and polyuria were considered DM rats (18).
Next, apomorphine (APO; Hubei Keyi Pharmaceutical Co., Ltd., Wuhan, China; 200301307) was applied to screen the DMED rats. Briefly, after being placed in a darkened, quiet room for 10 min, the DM rats were subcutaneously injected with APO (100 µg/kg) in the loose skin of the neck. The status of the rats in the environment described above was then monitored for 30 min using a video camera (Nikon, Tokyo, Japan; D7500). The growth or swelling of the penis with congestion or exposure of the glans was regarded as an erection, and DM rats that did not display penile erection and a level of fasting blood glucose >7.2 mmol/L were identified as DMED rats (7). Ultimately, 24 DMED rats were successfully established.
Grouping and administration
Following successful establishment of the DMED model, the 24 DMED rats were randomized into model control (MC), metformin, GLP low dose (GLP-L), and GLP high dose (GLP-H) groups (n=6) based on the number random table method. Subsequently, the rats in the metformin, GLP-L, and GLP-H groups were given 20 mg/kg metformin aqueous solution (Yuekang Pharmaceutical Group Co., Ltd., Beijing, China; 14870308) and 100 or 400 mg/kg GLP aqueous solution (Shanghai Yuanye Biotechnology, Ltd., Shanghai, China; F11HK145723), respectively (19,20). The rats in the other groups were given an equal volume of saline.
All treatments were administered daily for consecutive 8 weeks via intragastric administration. Additionally, all rats were fed a normal diet during the experiments, and both the weight and blood glucose of the rats were recorded weekly. According to the humane endpoints, the rats were observed continuously; rats that displayed a remarkable reduction in activity, emaciation, and abnormal diet were to be anesthetized and sacrificed immediately. However, no rats reached the humane endpoint criteria during the study.
Assessment of erectile function
Until the final administration, all rats were kept in a dimly-lit and silent environment, which was beneficial for observation. Thereafter, the rats were injected with APO (100 µg/kg) via the neck. Finally, the number and latency of erection within 0.5 h were monitored and documented. Erection latency was defined as the time difference between APO injection and erection.
Measurement of mean arterial pressure (MAP) and intracavernous pressure (ICP)
The rats were anesthetized via intraperitoneal tiletamine (50 mg/kg) injection and immobilized in the supine position. Next, a median incision was made along the neck to identify the right-side carotid artery, and a PE-50 tube filled with heparinized saline (250 IU/mL) was inserted to measure the MAP. Subsequently, a lower abdominal incision was made to find the CC and a 23-gauge butterfly needle, filled with heparin solution (250 U/mL) and connected to a pressure transducer, was inserted into the penile crus for the evaluation of ICP. Thereafter, the cavernous nerve (CN) was stimulated using an electrical stimulator with the following parameters: 10 V, 0.5 ms, and 0.5 mA (21). CN stimulation was carried out at least three times, with an interval of more than 10 min. During CN stimulation, the peak ICP of the CN was scaled with the help of an isometric force transducer, and the value of ICP/MAP was subsequently recorded.
Sample collection and storage
All rats were euthanized upon completing the MAP/ICP measurements, and their blood and penile tissues were collected. The blood samples were centrifuged for 15 min at 3,500 r/min, and the supernatants were then obtained and preserved at −80 ℃. Beyond that, the harvested penises were assigned into three portions: one was dissected for collecting the CC tissues, one was kept in paraformaldehyde (4%) for 24 h and embedded in paraffin, and one was stored at −80 ℃ for follow-up experiments.
Enzyme-linked immunosorbent assay (ELISA)
The stored penile tissues were ground into powder by liquid nitrogen and then homogenized in 340 µL phosphate-buffered saline (PBS). Next, the sample buffer was reacted with 20 µL 1 N HCl for 1 h, and PDEs were added to stop the reaction; thereafter, 20 µL 1 N was used to neutralize the HCl. Subsequently, the testosterone (T) (MM-0577R2), luteinizing hormone (LH) (ml470613), and follicle-stimulating hormone (FSH) (ml403115) contents in serum and cGMP (ml003133) content in penile tissues were determined by respective commercial kits according to the manufacturer’s protocol. FSH, LH, and cGMP ELISA kits were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd. (Shanghai, China), and the T ELISA kit was purchased from MEIMIAN (Jiangsu, China).
Measurement of oxidative stress levels
The CC tissues were taken out and washed with saline at 4 ℃. After absorbing the residual liquid with filter paper, 100 mg of CC tissues were cut and made into 10% homogenate. Hereafter, the levels of malondialdehyde (MDA), glutathione (GSH) and superoxide dismutase (SOD) in the CC tissues were measured according to the instructions.
Hematoxylin-eosin (HE) and Masson staining
The paraffin-embedded CC tissues were first sliced into 5-µm-thick serial sections using a microtome. Subsequently, the sections were dewaxed using xylene, rehydrated with 100% to 75% ethanol, and incubated with HE (Servicebio, Wuhan, China; G1003) or Masson (Servicebio, Wuhan, China; G1006) staining in strict accordance with the respective instructions. To evaluate the penile pathological changes, the sections stained with HE or Masson were observed using a light microscope (Nikon, Tokyo, Japan; E100). Additionally, collagen fiber/muscle fiber (CF/MF) was calculated by a blinded investigator. The MF and CF of the CC appeared in red and blue under a light microscope, respectively.
TUNEL staining
The penile tissues embedded in paraffin were cut into 5 µm-thick sections. Thereafter, tissue sections were dewaxed in xylene and rehydrated by graded ethanol, followed by treatment with Proteinase K (Servicebio, G1205) for 22 min at room temperature (RT) for antigen retrieval. Next, TUNEL reaction buffer (Servicebio, G1501) was used to incubate with the slices for 2 h at RT. After rinsing, the slides were counterstained using 4',6-diamidino-2-phenylindole (DAPI) (Servicebio, G1012) to observe the nuclei. Finally, the number of apoptotic cells was imaged and counted using a fluorescence microscope (Nikon, Tokyo, Japan; Eclipse C1).
Immunohistochemistry
Firstly, 5 µm-sections were prepared from paraffinized penile tissues, dewaxed in xylene, and rehydrated by ethanol. Next, endogenous peroxidase was blocked by treatment with 3% H2O2 for 10 min. Antigen retrieval was carried out by covering with 0.1 mol/L citrate buffer for 10 min, followed by incubating in 5% bovine serum albumin (BSA) for 1 h. Subsequently, the slices were probed with primary antibodies against caspase-3 (AF6311, 1:100), caspase-9 (AF6348, 1:100), and eNOS (AF0096, 1:200) overnight at 4 °C. After rinsing, horseradish peroxidase (HRP)-conjugated secondary antibodies (Abcam, Shanghai, China; ab97080, 1:1,000) were added to the sections for incubation at RT for 20 min. Hereafter, 3,3'-diaminobenzidine (DAB) was applied for visualization. Upon counterstaining with hematoxylin, the sections were examined by microscopy. ImageJ software (NIH, Bethesda, USA) was employed to quantify the caspase-3, caspase-9, and eNOS-positive cells. All of the primary antibodies were purchased from Affinity (Shanghai, China).
Detection of MMP
The harvested penile tissues were rinsed with pre-cooled PBS. Next, the tissue was sliced into blocks and placed into a pre-chilled glass homogenizer with mitochondria isolation buffer (containing a protease inhibitor) and homogenized on ice for about 10 strokes. After that, the homogenate was centrifuged twice to obtain the mitochondrion. Next, 90 µL of JC-1 working solution (five-fold-diluted) was used to incubate 10 µL of purified mitochondria. The red JC-1 polymer and green JC-1 monomer were detected using a fluorescence microplate reader (MD, San Francisco, USA; CMaxPlus) at the excitation/emission of 525/590 and 490/530 nm, respectively. The MMP was evaluated by the value of red/green fluorescence. Both the mitochondria isolation buffer (C3606) and JC-1 working solution (C2006) were supplied by Shanghai Biyuntian Biotechnology, Ltd. (Shanghai, China).
Quantitative polymerase chain reaction (qPCR)
CC tissues were first homogenized and then the total mRNA from the CC tissues was extracted by Trizol (Sangon Biotech Co., Ltd., Shanghai, China; B511311). Next, cDNA was synthesized using an RNA reverse-transcription kit (CWBIO, Beijing, China; CW2569) as per the general protocols. Thereafter, SYBR Premix Ex TaqII (Takara, Dalian, China; RR820A) was employed to perform qPCR. The relative mRNA expressions of NOS and TGF-β1 were tested, and GAPDH acted as a housekeeping gene. The qPCR primer sequences used in this study are shown in Table 1.
Table 1
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Rat eNOS | GCGGAGCAGAGCGGCCTTAT | TTTGGTGGGAGGACCGAGGG |
| Rat nNOS | TTCTGCTATCCGTTGTTGAATAGG | CACTGTCATAGCTGAGGTCTACCAA |
| Rat iNOS | TTCACGACACCCTTCACCACAA | CCATCCTCCTGCCCACTTCCTC |
| Rat TGF-β1 | GTCCAACATGATCGTGCGCT | CTTTAATAGCCCGCAGGTGG |
| Rat GAPDH | CGAGACACGATGGTGAAGGT | TGCCGTGGGTGGAATCATAC |
qPCR, quantitative polymerase chain reaction.
Western blotting
The total protein of the CC tissues was prepared with radioimmunoprecipitation assay (RIPA) buffer. A bicinchoninic acid (BCA) assay then was applied to estimate the protein concentration. Subsequently, the protein samples were separated by 5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. After blocking in 5% skimmed milk, the membranes were incubated at 4 ℃ overnight with primary antibodies against ERK1/2 (1:1,000, BF0412), p-ERK1/2 (1:1,000, AF1015), eNOS (1:1,000, AF0096), p-eNOS (Ser-1177) (1:1,000, AF3247), arginase II (1:1,000, DF3792), JNK (1:1,000, AF6318), p-JNK (1:1,000, AF3318), and GADPH (1:5,000, AF7021), followed by co-incubation for another 1.5 h with HRP-conjugated secondary antibody. Finally, the blots were developed using an electrochemiluminescence (ECL) reagent and quantified with ImageJ software. All of the primary antibodies were purchased from Affinity.
Statistical analysis
The data in this study were presented as mean ± standard deviation and analyzed using SPSS 16.0 (SPSS Inc., Chicago, USA). The sample size was decided based on previous studies. The investigators were blinded to group allocations in all experiments. One-way analysis of variance (ANOVA) and the Tukey test were applied for multi-group comparisons. The Kruskal-Wallis H test was applied if variances were not equal. P<0.05 was considered a statistically significant difference.
ResultsOther Section
GLP restored the body weight and reduced blood glucose in DMED rats
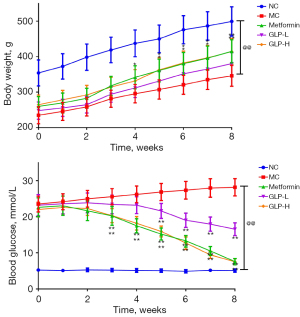
As depicted in Figure 1, relative to the NC group, rats in the MC group had a significant downregulation in bodyweight and upregulation in blood glucose (P<0.01). However, during the treatment process, the DMED rats regained their weight progressively from week 4 or 5 after treatment with metformin or high doses of GLP (P<0.05). In addition, the blood glucose levels of rats in both the metformin and GLP-H groups were markedly reduced from week 3, and rats in GLP-L had reduced blood glucose from week 5 (P<0.01).
GLP improved the erectile function of DMED rats
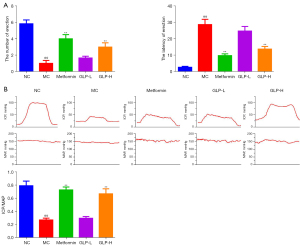
Subsequently, we recorded the number and latency of erection as well as the value of ICP and ICP/MAP to assess the effect of GLP on ED. As exhibited in Figure 2A, we observed that DMED rats had significantly lower numbers of erections and longer erection latency than normal rats (P<0.01), but this situation was improved after treatment with metformin or high dose of GLP for 8 weeks (P<0.05). Similarly, there was an evident decrease in ICP and ICP/MAP in the MC group (P<0.01); however, after 8 weeks of treatment, the DMED rats exhibited a notable increase in the value of ICP and ICP/MAP (P<0.01), albeit still lower than NC group (Figure 2B).
GLP recovered the T and cGMP levels in DMED rats
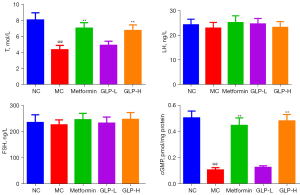
In the next step, we performed ELISA to evaluate the effect of GLP in regulating the level of sexual hormones (including T, LH, and FSH) as well as cGMP. As shown in Figure 3, we observed that the content of T and cGMP in the MC group was significantly lower than that in the NC group (P<0.01). However, both metformin and GLP (400 mg/kg) treatments remarkably elevated the content of T and cGMP (P<0.01). In addition, the level of LH and FSH undulated in a small extant in the NC and MC groups, and GLP did not impact the variations (P>0.05).
GLP down-regulated MDA content but up-regulated GSH and SOD contents in DMED rats
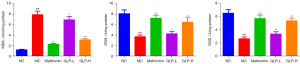
Hereafter, we measured the contents of MDA, GSH and SOD in the CC. As presented in Figure 4, in comparison with the rats in the NC group, MC rats had a higher MDA content as well as lower GSH and SOD contents. However, the situations were significantly reversed following metformin and GLP (both low- and high-dose) treatment (P<0.05).
GLP improved the histological injury of the CC in DMED rats
Based on the aforementioned findings, the histological changes of the CC were evaluated after 8 weeks of treatment. Figure 5 exhibits representative images of the HE and Masson staining. The HE staining displayed the CC damage in DMED rats, including the expanded septum of the CC, as well as the cluttered distribution of blood sinusoid, endothelial cells, and smooth muscle cells in the CC. These histopathological alterations were effectively improved after treatment with metformin or GLP (both low- and high-dose).
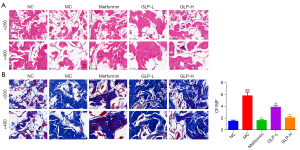
Masson staining showed that the distributions of MF and CF were disordered and uneven, and some smooth muscles were bifurcated, broken, and thinned in DMED rats. Apart from these, the value of MF/CF was remarkably enhanced in DMED rats (P<0.01). However, GLP treatment showed notable amelioration of the aforementioned changes, both in low and high doses (P<0.01).
GLP prevented the increase in cell apoptosis in DMED rats
Given that cell apoptosis of the penile tissue is one of the major events causing DMED, we investigated the role of GLP on cell apoptosis in the penile tissue of DMED rats by TUNEL assay. As illustrated in Figure 6A, the rate of TUNEL-positive cells was increased in the DMED rats (P<0.01). However, metformin as well as high- and low-dose GLP markedly attenuated the upregulated rate of TUNEL-positive cells (P<0.01).
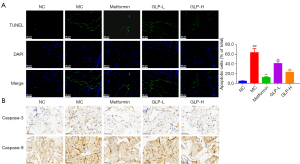
Furthermore, the accumulation of caspase-3 and caspase-9 was observed by immunohistochemistry. In contrast to the NC group, caspase-3 and caspase-9 accumulated significantly in the DMED rats. Treatment with metformin or high-dose GLP induced a substantial reduction in these apoptosis-associated proteins (P<0.05; Figure 6B).
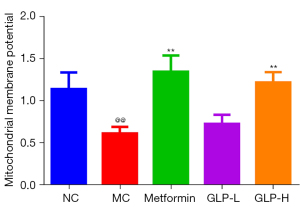
GLP ameliorated the decrease in MMP in DMED rats
Next, we measured MMP to evaluate the effect of GLP on mitochondrial function. In comparison with the NC group rats, rats in the MC group had a lower MMP (P<0.01), but treatment with metformin or high-dose GLP markedly reversed the decrease in MMP in DMED rats (P<0.01; Figure 7).
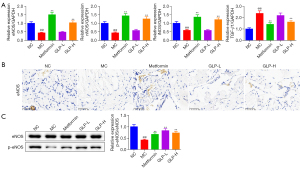
GLP enhanced the expression of NOS and TGF-β1 in DMED rats
Subsequently, qPCR was performed to detect the effect of GLP on the mRNA expression of NOS and TGF-β1. As displayed in Figure 8A, the NOS mRNA level of the rats was dramatically reduced, while that of TGF-β1 mRNA was remarkably increased under DMED conditions (P<0.01). In contrast, there was a substantial increase in NOS mRNA expression and a dramatic decrease in TGF-β1 mRNA expression after treatment with metformin or high-dose GLP for 8 weeks (P<0.01). Consistent with the qPCR results, the immunohistochemistry findings of eNOS (Figure 8B) and western blot analysis of eNOS phosphorylation (Figure 8C) also showed a similar trend.
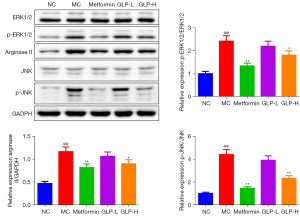
GLP suppressed the phosphorylation of ERK1/2 and JNK and protein expression of arginase II in the DMED rats
To further investigate the possible mechanism of GLP on DMED, western blotting was carried out to test the phosphorylation of ERK1/2 and JNK as well as the expression of arginase II. The results indicated that the phosphorylation of ERK1/2 and JNK and the expression of arginase II were remarkably upregulated in the DMED rats (P<0.01), and metformin or high-dose GLP treatment effectively downregulated the phosphorylation of ERK1/2 and JNK as well as the expression of arginase II (P<0.05; Figure 9).
DiscussionOther Section
GLP, a key pharmacodynamic constituent of Ganoderma, is well-known for its various pharmacological properties (22). GLP contains a substantial amount of glucose, based on the database of traditional Chinese medicine systems pharmacology (TCMSP; https://old.tcmsp-e.com/tcmsp.php), the glucose of the GLP can be used to treat hypertension, DM, obesity and so on, and iNOS as well as eNOS were predicted as the related targets. A previous study demonstrated that GLP exerts an anti-hyperglycemic function, and the probable mechanism may be related to stimulation of the FAM3C/HSF1/CaM pathway (23). Additionally, GLP can alleviate metabolic abnormalities, and prevent the development of diabetic renal complications in streptozotocin-induced DM mice (24). Furthermore, research conducted by Pan et al. revealed that a proteoglycan extracted from Ganoderma lucidum can not only manage blood glucose well in DM mice, but can also be used as a new agent for the protection of pancreatic β-cells against oxidative stress during diabetes treatment (25). In this study, we observed that GLP treatment ameliorated ED in DMED rats, which might relate to the inhibition of apoptosis, the upregulation of NOS, as well as regulation of the ERK/JNK signaling pathway.
Fibrosis of the CC is considered to assist in the progression of DMED, which is characterized by CF accumulation and MF reduction in the CC (26). Multiple factors, such as prolonged hyperglycemia and penile nerve damage, will lead to CC fibrosis in DM patients (17). Furthermore, TGF-β1 is an important fibrotic cytokine that can induce fibrosis in the CC and enhance the transformation of epithelial interstitial and extracellular matrix products (27). A previous study has confirmed that GLP may act as a natural and promising anti-fibrotic agent (28). Consistently, in the present study, we detected the mRNA levels of TGF-β1 by qPCR and measured CF and MF by Masson staining, and found that the mRNA level of TGF-β1 and the value of CF/MF in DMED rats was significantly decreased with GLP treatment, which provides direct evidence that GLP could improve DMED by suppressing fibrosis of the CC.
Apoptosis, an orderly and spontaneous form of cell death that is regulated by genes, is an essential pathophysiological process that maintains the proper functioning of cells and organs (29). Furthermore, apoptosis is also a pivotal pathological mechanism of ED, which may contribute to ischemia in the CC, thereby diminishing erectile function (30). Zhang et al. observed that there is a substantial increase in the apoptosis index of DMED rats (31). Moreover, research has demonstrated that the erectile function is improved in DM rats after inhibiting oxidative stress, fibrosis, and apoptosis in the CC (6). As expected, in our study, GLP effectively lowered the apoptotic cell percentage in the penile tissue of DMED rats.
Members of the caspase family are regarded as crucial mediators for mitochondrial-mediated apoptosis, among which caspase-3 and caspase-9 are representative executors (32). Activated caspase-9 triggers the activation of caspase-3, which in turn cleaves its protein substrate and finally induces apoptosis (33). In a cellular model of non-small cell lung cancer, researchers found that GLP can promote apoptosis by regulating the expressions of Bcl-2, Bax, cleaved PARP, and cleaved caspase-3 (34). Furthermore, another study also reported that GLP-induced tumor apoptosis was related to the regulation of caspase-3, caspase-9, Bcl-2, and Bax2 (35). In this study, we detected the status of MMP and the protein expressions of caspase-3 and caspase-9 in penile tissue and found that GLP treatment increased MMP and impaired the expressions of caspase-3 and caspase-9 in DMED rats, which indicated that GLP could improve fibrosis of the CC by suppressing apoptosis.
NOS is a kind of NO-generating enzyme with three isoforms: nNOS, iNOS, and eNOS (36). Recently, an increasing number of studies have demonstrated that NO is a critical neurotransmitter in the CC for the mediation of penile erection (37,38). Consequently, improving NOS activity in the penile CC is an efficient approach for the treatment of ED. Wen et al. revealed that GLP exerts potent anti-inflammatory activity by suppressing the excessive pro-inflammatory cytokines and NO production in lipopolysaccharide (LPS)-stimulated cells (15). Our results verified that the mRNA and protein levels of NOS in DMED rats tended to be similar to the normal rats after GLP intervention for 8 weeks, implying that GLP could attenuate DMED by upregulating the expression of NOS.
DM is a systemic metabolic and immune disease that can lead to significant changes in multiple systems (39). The ERK/JNK pathway is invariably activated when DM occurs, which plays a vital role in apoptosis (9). A previous study revealed that inhibition of the ERK1/2 pathway can ameliorate DM complications (atherosclerosis) in vivo (40). In addition, Yung and Giacca found that JNK is a latent target for type 2 diabetes treatment due to its regulation of insulin resistance as well as β cell dysfunction (41). Furthermore, GLP has been shown to suppress DNA synthesis, ERK and JNK phosphorylation, and apoptosis in HuH-7 cells (42). In this study, we observed that GLP decreased the phosphorylation of ERK and JNK in DMED rats, providing novel evidence for the relationship between the ERK/JNK pathway and DEMD.
All the same, the present study also has some limitations. The lack of cellular experiments is the major defect of the present study. In addition, we didn’t use NOS inhibitors or ERK/JNK pathway agonists to further confirm the mechanism of GLP in DMED. Therefore, in the future, we will continue to validate the function and mechanism of GLP in improving DMED from a cellular aspect and using NOS inhibitor as well as ERK/JNK pathway agonist. In summary, GLP may ameliorate the erectile function of DMED rats by repairing the pathological damage of the CC, upregulating the expression of NOS, decreasing mitochondrial-mediated apoptosis, as well as via the ERK/JNK pathway. This was a pivotal finding that might be applicable to other mammalian species or even to other vertebrate groups. Furthermore, our study provides a potential mechanism for the treatment of DMED with GLP, which increases the scientific basis for its clinical application.
AcknowledgmentsOther Section
Funding: None.
FootnoteOther Section
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-428/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-428/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-428/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were performed with the approval of the Animal Experimentation Ethics Committee of Zhejiang Eyong Pharmaceutical Research and Development Center (Approval No. ZJEY-20211008-01), and were conducted according to the institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Ma WJ, Qin M, Cui TW, et al. Relationship between the risk factors of cardiovascular disease by testing biochemical markers and young men with erectile dysfunction: a case-control study. Transl Androl Urol 2021;10:724-33. [Crossref] [PubMed]
- Wang X, Zhang F, Guo L, et al. Significance of hyperhomocysteinaemia as an effective marker for vasculogenic erectile dysfunction: a cross-sectional study. Transl Androl Urol 2022;11:397-406. [Crossref] [PubMed]
- Wang L, Wang F, Zhao L, et al. Mesenchymal Stem Cells Coated by the Extracellular Matrix Promote Wound Healing in Diabetic Rats. Stem Cells Int 2019;2019:9564869. Erratum in: Stem Cells Int 2019;2019:9581478. [PubMed]
- Li X, Zhao Q, Wang J, et al. Efficacy and safety of PDE5 inhibitors in the treatment of diabetes mellitus erectile dysfunction: Protocol for a systematic review. Medicine (Baltimore) 2018;97:e12559. Erratum in: Medicine (Baltimore) 2018;97:e13203. [Crossref] [PubMed]
- Ausó E, Gómez-Vicente V, Esquiva G. Visual Side Effects Linked to Sildenafil Consumption: An Update. Biomedicines 2021;9:291. [Crossref] [PubMed]
- Li H, Xu W, Liu X, et al. JAK2 deficiency improves erectile function in diabetic mice through attenuation of oxidative stress, apoptosis, and fibrosis. Andrology 2021;9:1662-71. [Crossref] [PubMed]
- Wang JS, Feng JL, Li X, et al. Effect of leech-centipede medicine on improving erectile function in diabetes-induced erectile dysfunction rats via PDE5 signalling pathway-related molecules. Pharm Biol 2021;59:167-74. [Crossref] [PubMed]
- Chen F, Xu Y, Wang J, et al. Relaxation Effect of Patchouli Alcohol in Rat Corpus Cavernous and Its Underlying Mechanisms. Evid Based Complement Alternat Med 2020;2020:3109069. [Crossref] [PubMed]
- Wu KM, Hsu YM, Ying MC, et al. High-density lipoprotein ameliorates palmitic acid-induced lipotoxicity and oxidative dysfunction in H9c2 cardiomyoblast cells via ROS suppression. Nutr Metab (Lond) 2019;16:36. [Crossref] [PubMed]
- Liu Z, Yu L, Gu P, et al. Surface-Engineered Cubosomes Serve as a Novel Vaccine Adjuvant to Modulate Innate Immunity and Improve Adaptive Immunity in vivo. Int J Nanomedicine 2020;15:8595-608. [Crossref] [PubMed]
- Zeng P, Guo Z, Zeng X, et al. Chemical, biochemical, preclinical and clinical studies of Ganoderma lucidum polysaccharide as an approved drug for treating myopathy and other diseases in China. J Cell Mol Med 2018;22:3278-97. [Crossref] [PubMed]
- Zeng P, Chen Y, Zhang L, et al. Ganoderma lucidum polysaccharide used for treating physical frailty in China. Prog Mol Biol Transl Sci 2019;163:179-219. [Crossref] [PubMed]
- Li L, Li RC, Song YH, et al. Effects of a Ganoderma atrum polysaccharide against pancreatic damage in streptozotocin-induced diabetic mice. Food Funct 2019;10:7227-38. [Crossref] [PubMed]
- Hassan HM, Mahran YF, Ghanim AMH. Ganoderma lucidum ameliorates the diabetic nephropathy via down-regulatory effect on TGFβ-1 and TLR-4/NFκB signalling pathways. J Pharm Pharmacol 2021;73:1250-61. [Crossref] [PubMed]
- Wen L, Sheng Z, Wang J, et al. Structure of water-soluble polysaccharides in spore of Ganoderma lucidum and their anti-inflammatory activity. Food Chem 2022;373:131374. [Crossref] [PubMed]
- Guo C, Guo D, Fang L, et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr Polym 2021;267:118231. [Crossref] [PubMed]
- Ding F, Shan C, Li H, et al. Simvastatin alleviated diabetes mellitus-induced erectile dysfunction in rats by enhancing AMPK pathway-induced autophagy. Andrology 2020;8:780-92. [Crossref] [PubMed]
- Wang J, Yao Y, Liu X, et al. Protective effects of lycium barbarum polysaccharides on blood-retinal barrier via ROCK1 pathway in diabetic rats. Am J Transl Res 2019;11:6304-15. [PubMed]
- Jin M, Zhu Y, Shao D, et al. Effects of polysaccharide from mycelia of Ganoderma lucidum on intestinal barrier functions of rats. Int J Biol Macromol 2017;94:1-9. [Crossref] [PubMed]
- Wu S. Hypolipidaemic and anti-lipidperoxidant activities of Ganoderma lucidum polysaccharide. Int J Biol Macromol 2018;118:2001-5. [Crossref] [PubMed]
- Kim KS, Bae WJ, Kim SJ, et al. Improvement of erectile dysfunction by the active pepide from Urechis unicinctus by high temperature/pressure and ultra - wave assisted lysis in Streptozotocin Induced Diabetic Rats. Int Braz J Urol 2016;42:825-37. [Crossref] [PubMed]
- Chen SD, Yong TQ, Zhang YF, et al. Inhibitory Effect of Five Ganoderma Species (Agaricomycetes) against Key Digestive Enzymes Related to Type 2 Diabetes Mellitus. Int J Med Mushrooms 2019;21:703-11. [Crossref] [PubMed]
- Pan R, Lou J, Wei L. Significant effects of Ganoderma lucidum polysaccharide on lipid metabolism in diabetes may be associated with the activation of the FAM3C-HSF1-CAM signaling pathway. Exp Ther Med 2021;22:820. [Crossref] [PubMed]
- He CY, Li WD, Guo SX, et al. Effect of polysaccharides from Ganoderma lucidum on streptozotocin-induced diabetic nephropathy in mice. J Asian Nat Prod Res 2006;8:705-11. [Crossref] [PubMed]
- Pan Y, Yuan S, Teng Y, et al. Antioxidation of a proteoglycan from Ganoderma lucidum protects pancreatic β-cells against oxidative stress-induced apoptosis in vitro and in vivo. Int J Biol Macromol 2022;200:470-86. [Crossref] [PubMed]
- Song J, Sun T, Tang Z, et al. Exosomes derived from smooth muscle cells ameliorate diabetes-induced erectile dysfunction by inhibiting fibrosis and modulating the NO/cGMP pathway. J Cell Mol Med 2020;24:13289-302. [Crossref] [PubMed]
- Liu K, Cui K, Feng H, et al. JTE-013 supplementation improves erectile dysfunction in rats with streptozotocin-induced type I diabetes through the inhibition of the rho-kinase pathway, fibrosis, and apoptosis. Andrology 2020;8:497-508. [Crossref] [PubMed]
- Luo Q, Di L, Dai WF, et al. Applanatumin A, a new dimeric meroterpenoid from Ganoderma applanatum that displays potent antifibrotic activity. Org Lett 2015;17:1110-3. [Crossref] [PubMed]
- Wang B, Han D, Li F, et al. Elevated IL-22 in psoriasis plays an anti-apoptotic role in keratinocytes through mediating Bcl-xL/Bax. Apoptosis 2020;25:663-73. [Crossref] [PubMed]
- Zhu GQ, Jeon SH, Bae WJ, et al. Efficient Promotion of Autophagy and Angiogenesis Using Mesenchymal Stem Cell Therapy Enhanced by the Low-Energy Shock Waves in the Treatment of Erectile Dysfunction. Stem Cells Int 2018;2018:1302672. [Crossref] [PubMed]
- Zhang H, Tong WT, Zhang CR, et al. Gross saponin of Tribulus terrestris improves erectile dysfunction in type 2 diabetic rats by repairing the endothelial function of the penile corpus cavernosum. Diabetes Metab Syndr Obes 2019;12:1705-16. [Crossref] [PubMed]
- Zhao S, Fan Z, Hu J, et al. The differential effects of isoflurane and sevoflurane on neonatal mice. Sci Rep 2020;10:19345. [Crossref] [PubMed]
- Xu J, Feng Z, Wang X, et al. hUC-MSCs Exert a Neuroprotective Effect via Anti-apoptotic Mechanisms in a Neonatal HIE Rat Model. Cell Transplant 2019;28:1552-9. [Crossref] [PubMed]
- Wang W, Gou X, Xue H, et al. Ganoderan (GDN) Regulates The Growth, Motility And Apoptosis Of Non-Small Cell Lung Cancer Cells Through ERK Signaling Pathway In Vitro And In Vivo. Onco Targets Ther 2019;12:8821-32. [Crossref] [PubMed]
- Song M, Li ZH, Gu HS, et al. Ganoderma lucidum Spore Polysaccharide Inhibits the Growth of Hepatocellular Carcinoma Cells by Altering Macrophage Polarity and Induction of Apoptosis. J Immunol Res 2021;2021:6696606. [Crossref] [PubMed]
- Suksawat M, Techasen A, Namwat N, et al. Inhibition of endothelial nitric oxide synthase in cholangiocarcinoma cell lines - a new strategy for therapy. FEBS Open Bio 2018;8:513-22. [Crossref] [PubMed]
- Coletto E, Dolan JS, Pritchard S, et al. Contractile dysfunction and nitrergic dysregulation in small intestine of a primate model of Parkinson's disease. NPJ Parkinsons Dis 2019;5:10. [Crossref] [PubMed]
- Li R, Cui K, Liu K, et al. Metabolic syndrome in rats is associated with erectile dysfunction by impairing PI3K/Akt/eNOS activity. Sci Rep 2017;7:13464. [Crossref] [PubMed]
- Russart KLG, Chbeir SA, Nelson RJ, et al. Light at night exacerbates metabolic dysfunction in a polygenic mouse model of type 2 diabetes mellitus. Life Sci 2019;231:116574. [Crossref] [PubMed]
- Shi LL, Hao M, Jin ZY, et al. Liraglutide Alleviates Diabetic Atherosclerosis through Regulating Calcification of Vascular Smooth Muscle Cells. Dis Markers 2022;2022:5013622. [Crossref] [PubMed]
- Yung JHM, Giacca A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020;9:706. [Crossref] [PubMed]
- Li CH, Chen PY, Chang UM, et al. Ganoderic acid X, a lanostanoid triterpene, inhibits topoisomerases and induces apoptosis of cancer cells. Life Sci 2005;77:252-65. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


