
Establishment of a novel prognostic prediction model through bioinformatics analysis for prostate cancer based on ferroptosis-related genes and its application in immune cell infiltration
Introduction
Prostate cancer (PCa) is one of the most prevalent male malignancies worldwide according to the latest cancer statistics for 2022 (1). For patients with localized PCa, 20–25% of patients may still experience biochemical recurrence (BCR) during follow-up despite surgery or other treatments, and severe cases may eventually progress to castration-resistant prostate cancer (CRPC) with increasing specific mortality and survival risk (2). Therefore, the development of new prognostic identification markers is crucial for monitoring the clinical survival status of PCa patients, especially high-risk PCa patients.
In 2012, in order to describe the morphology of cell death induced by erastin, a small molecule binds and inhibits voltage-dependent anion channels, Dixon et al. coined the term ferroptosis. Ferroptosis is iron ion-catalyzed necrotic cell death induced by inhibiting cysteine import, resulting in glutathione depletion and inhibition of glutathione peroxidase 4 (GPX4) (3). In recent years, studies have reported that ferroptosis regulates the progression of various cancers by inhibiting tumor cell proliferation. Whether the process is genetically determined or pathologically triggered remains unclear. Under the catalysis of divalent iron or ester oxygenase, the highly expressed unsaturated fatty acids on the cell membrane undergo lipid peroxidation and lipid-reactive oxygen accumulation, leading to cell ferroptosis (4). Currently, the role of ferroptosis in tumor development, treatment, and prognosis has become an active area of research. PCa is considered one of the diseases associated with ferroptosis (5). Dyslipidemia was demonstrated to be the most commonly acquired metabolic phenotype in androgen receptor inhibitor (ARI)-resistant cells. In the treatment of CRPC, adjusting the ratio of saturated to unsaturated phospholipids increases the sensitivity of CRPC cells to ferroptosis and increases cytotoxicity (6). Erastin and the GPX4 inhibitor RSL3, 2 ferroptosis inducers (FINs), have been shown to slow tumor cell growth in vivo and significantly reduces tumor cell migration in vitro, suggesting that treatment-resistant PCa cells are highly sensitive to these 2 iron death inducers (5). Bordini et al. confirmed that androgen receptor-positive PCa cell lines VCaP and LNCaP have high iron sensitivity and eventually cause cell membrane protein damage, suggesting that ferroptosis is associated with poor prognosis in prostate tumors. Furthermore, in vitro experiments confirmed that bicalutamide-iron conjugate resulted in protein oxidation and successfully inhibited tumor expansion, whereas the single compound was ineffective (7). The above studies have confirmed that ferroptosis may become an entry point for the prognosis of PCa survival.
In the past decade, cancer immunotherapy research has made historic breakthroughs (8). Tumor microorganisms act on the tumor-associated immune microenvironment (TAIM) by inducing immunogenic cell death, assisting pattern recognition receptor-mediated signaling pathways, and bacterial antigens mimicking tumor antigens, which are of great significance for tumor development and immunotherapy response (9). A suppressive TAIM, characterized by immune cell and stromal cell infiltration, severely affects tumor proliferation, metastasis, recurrence, and immunotherapy resistance, as seen in studies of a variety of cancers (10). Based on the TCGA database, a variety of different immune cells can be detected from PCa tissue (11). So far, the pattern of immune cell infiltration (ICI) and clinical outcome in PCa are poorly characterized. An in-depth understanding of the TAIM is urgently needed to identify the PCa subtypes that contribute to differential prognosis and lay the groundwork for improved immunotherapy.
The above evidence shows that the expression of ferroptosis-related genes (FRGs) and ICI are closely related to the local growth and metastasis of PCa, which may has certain significance for predicting the individualized health prognosis of patients. For the first time, this study evaluated the individual status of PCa patients from multiple perspectives by constructing a prognostic characteristic model of overall survival (OS) and disease-free survival (DFS) with the combination of analysing the immune infiltration landscape analysis, in order to supplement the existing clinical staging system and promote the precision of cancer treatment. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-454/rc).
Methods
Based on bioinformatics analysis, this study firstly searched for FRG templates and screened out the genes in the group. Through univariate, LASSO, and multivariate regression analysis, a predictive model was constructed, and then the model was varified by means of clinicopathological samples; then the application of FRGs in immune infiltration was tested.
Data acquisition
A total of 268 FRGs were extracted from previous study (12). After intersecting these genes with the expression matrix of PCa transcriptome data in The Cancer Genome Atlas (TCGA; https://tcga-data.nci.nih.gov/tcga/), a total of 249 genes were involved in the follow-up analysis.
PCa patient cohort data (ReadCounts) were downloaded from TCGA database (https://portal.gdc.cancer.gov/) and be served as a training set. The RNA-Seq expression matrix of 500 patients was obtained using the R package TCGAbiolinks. For the purpose of obtaining comparable standardized databases, fragments per kilobase of transcript per million fragments mapped (FPKM) values of RNA-Seq were log2 transformed. Finally, 498 PCa tissues and 52 adjacent normal tissues were obtained. The clinical information of 500 patients with PCa was collected (Table 1). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Table 1
| Patients characteristic | Number (%) |
|---|---|
| Age (years) | |
| ≥65 | 167 (33.4) |
| <65 | 333 (66.6) |
| T stage | |
| T1/T2 | 188 (38.1) |
| T3/T4 | 305 (61.9) |
| N stage | |
| N0 | 348 (81.5) |
| N1 | 79 (18.5) |
| Gleason score | |
| ≥7 | 455 (91.0) |
| <7 | 45 (9.0) |
PRAD, prostate adenocarcinoma.
Discernment of differentially expressed fRGs between PCa and adjacent non-tumor tissue
Differential expression analysis of FRGs in PCa tissues and adjacent non-tumor tissues was performed utilizing the Limma package in R, with thresholds of |log2 fold change (FC)| >1. FDR <0.05 was considered statistically significant. The results were presented in 3 forms: heat map, volcano plot, and boxplot.
Enrichment analysis
Subsequently, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was forthputte with R package clusterProfiler. The results were represented by dot plots.
Construction and validation of a prediction model based on FRGs
To identify OS- and DFS-related FRGs in PCa, we firstly executed univariate Cox analysis to initially screen for statistically significant genes. Least absolute shrinkage and selection operator (LASSO) was processed so as not to overfit and remove tightly correlated genes. Subsequently, we used multivariate Cox regression with the R package Glmnet to build the OS- and DFS-related prediction model. The formula of the risk score was as follows:
where Coefm represents the risk coefficient and Expm represents the relative mRNA expression of each FRG. We stratified patients with PCa into high-risk and low-risk group based on median risk scores obtained from database data. Survival status was assessed by the Kaplan-Meier (K-M) method. Sensitivity and specificity of survival predictions were tested by receiver operating characteristic (ROC) analysis using the R package survivalROC. The area under the ROC curve (AUC) was calculated as an indicator of the accuracy of the predictions for both groups. The K-M curves of each single gene in the OS and DFS prediction model were analyzed separately to assess their correlation. Finally, we compared the FRGs in the OS and DFS prediction model with the clinicopathological parameters of PCa patients.
Relationships of FRGs with ICI
Single-sample gene set enrichment analysis (ssGSEA) was used to quantify the relative infiltration of immune cell types in the tumor microenvironment. The unique genomes of each independent immune cell subsets were obtained from the KEGG (https://www.genome.jp/kegg/) database. Gene set variation analysis (GSVA) was implemented with the help of R package GSVA to assess the relative abundance of immune cells between different risk groups. To standardize the data, 1 was set as the maximum score and 0 was set as the minimum score.
The CIBERSORT (https://cibersort.stanford.edu/) algorithm was used to assess the richness of ICI in different groups of patients. The Estimate algorithm was used to obtain the difference of Stromasignature and ImmuneSignature between high-risk group and low-risk group.
Statistical analysis
All data analysis was performed using R4.1.1 (https://cran.r-project.org). Survival curves for OS- and DFS-prognostic analysis were produced by the K-M method, and the log-rank test was used to acknowledge the distinguishment of differences. Univariate and multivariate Cox regression models were used to calculate hazard ratios (HR), and forest plots visualized the coefficients of these regression models. LASSO regression was applyed to avoid overfitting during the construction of the final prediction model. Continuous variables were compared between the 2 groups by the Wilcoxon rank test. Comparisons of categorical variables between 2 groups were performed by the chi-square test. The Kruskal-Wallis test was used to compare differences among 3 or more groups. As for ROC, models with AUC greater than 0.75 perform better. Statistical significance was defined as |log2 FC >1.0| and adjusted FDR P value <0.05.
Results
Differentially expressed FRGs between PCa and adjacent non-tumor tissues
A total of 500 patients with primary localized PCa containing complete RNA-Seq transcript data and clinical follow-up information including age, tumor stage, and gleason score were enrolled in this study. Among the 249 FRGs included in the study, 31 were differentially expressed (Figure 1A).
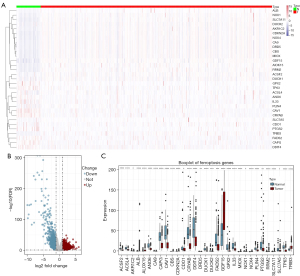
Volcano plots and boxplots were generated to demonstrate the expression patterns of FRGs differentially expressed between PCa and adjacent normal tissues (Figure 1B,1C).
GO and KEGG enrichment analysis of differentially expressed FRGs
GO (http://geneontology.org) and KEGG (https://www.genome.jp/kegg/) enrichment analysis was performed based on differentially expressed FRGs. According to the results of GeneRatio, most of the GO terms were enrich for several metabolism processes, such as reactive oxygen species metabolic process, fatty acid metabolic process, and response to oxidative stress. Interestingly, the KEGG terms were closely associated with microRNAs in cancer, ferroptosis, arachidonic acid metabolism, and thyroid synthesis hormone. Dot plots were used to show the relationship between FRGs and GO and KEGG enrichment analysis (Figure 2A,2B).

Establishment and validation of OS and DFS models in PCa patients based on FRGs
In the initial anlaysis by univariate Cox regression, a total of 31 FRGs were significantly correlated with OS, and 6 of them (AKR1C1, MT3, SLC2A6, AURKA, AKR1C3, TFRC) were shown in the forest plot (Figure 3A). Hub genes were selected using LASSO to screen the colinearity and reduce the risk of overfitting simultaneously. The LASSO Cox regression method constructed a 21-mRNA signature (Figure 3B,3C).
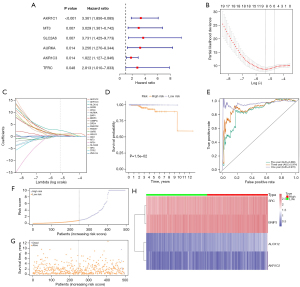
In addition, 4 genes, ALOX12, AKR1C2, SRC, and BNIP3, were identified as predictors and confirmed in the multivariate Cox regression analysis to establish a prediction model: OS-related prediction model = (2.5213096 × expression value of ALOX12 + (0.9157942 × expression value of AKR1C2) + (1.106994 × expression value of SRC) + (0.7573778 × expression value of BNIP3). Among them, 2.5213096, 0.9157942, 1.106994 and 0.7573778 are the distribution coefficients of the predictors ALOX12, AKR1C2, SRC and BNIP3 in the model, respectively.
Subsequently, according to their median risk score, we divided all the cases in The Cancer Genome Atlas-Prostate Adenocarcinoma (TCGA-PRAD) cohort into high- and low-risk groups. The K-M curve indicated that the high-risk group had a lower OS compared with the low-rank group (P=0.015) (Figure 3D).
When evaluating the OS-related prediction, the 1-, 3-, and 5-year AUCs of the developed gene signature were 0.970, 0.914, and 0.866, respectively, all of which were significant (Figure 3E). Figure 3F,3G show the distribution of OS-related prediction models for patients in TCGA dataset, and it was evident that the OS-related prediction model were able to successfully differentiate between high-risk and low-risk groups.
Figure 3H shows the correlations between the 4 genes ALOX12, AKR1C2, SRC, and BNIP3 in the high- and the low-risk groups. In order to explore the correlations between these 4 genes and the prognosis of PCa patients, survival analysis was performed. High expression of SRC was significantly correlated with poorer OS in the K-M curve (P=0.00358) (Figure 4). In addition, the genes ALOX12 (P=0.07935), AKR1C2 (P=0.19687), and BNIP3 (P=0.19119) were not significantly correlated with the OS rate of PCa patients (Figure 4).

The univariate Cox regression method was first to use to identify the 249 FRGs that were associated with DFS (Figure 5A). Hub genes were then selected using the LASSO method to minimize the risk of overfitting (Figure 5B,5C). The multivariate Cox analysis was performed to determine the real DFS-related factors and finally identified a prognostic panel of 8 genes (ATG16L1, AKR1C2, MIOX, ALOX12, DRD4, IDH1, RELA, CHMP6), and a prediction model was established: DFS-related prediction model = (0.32010082 × expression value of ATG16L1) + (0.75803321 × expression value of AKR1C2) + (0.76159814 × expression value of MIOX) + (2.77524618 × expression value of ALOX12) + (0.97252682 × ex1-9pression value of DRD4) + (0.07452585 × expression value of IDH1) + (1.74889181 × expression value of RELA) + (−1.10159123 × expression value of CHMP6). Subsequently, we divided the 500 PCa cases into high- and low-risk groups according to the median of the DFS-related prediction model. The K-M plot indicated that the DFS rate of patients in the high-risk group was significantly lower than that of patients in the low-risk group (P=0.01) (Figure 5D).
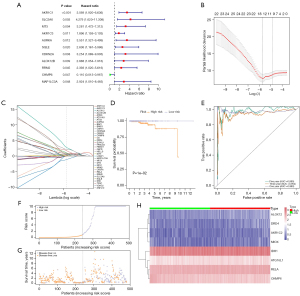
Moreover, the AUC for 1 year, 3 years, and 5 years DFS were 0.980, 0.954, and 0.990, respectively, which were meaningful classification results (Figure 5E). Figure 5F,5G show the distribution of DFS-related prediction models for patients in TCGA dataset. Figure 5H shows the correlations of 8 genes between high-risk and low-risk groups.
In order to explore the correlations between these 8 genes and the DFS rate of PCa patients, they were subjected to survival analysis. The results showed that ATG16L1 (P=0.06234), AKR1C2 (P=0.32121), MIOX (P=0.86353), ALOX12 (P=0.11591), DRD4 (P=0.31457), IDH1 (P=0.55412), RELA (P=0.31046), and CHMP6 (P=0.09613) were not significantly associated with the DFS rate of PCa patients (Figure 6).

Validation of predictive models using clinicopathological parameters from high- and low-risk patients
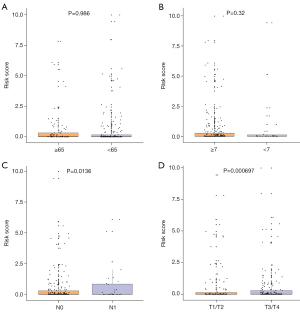
The OS-related prediction model showed statistically significant higher values for T3–4 than for T1–2 (P=0.0057), while no differences in OS-related prediction model values were observed between N0 and N1 (P=0.283), age ≥65 and age <65 (P=0.94), and Gleason score ≥7 and <7 (P=0.756) (Figure 7).

The DFS-related prediction model was significantly different between T3–4 than T1–2 (P<0.001), N0 than N1 stage (P=0.0136); while no differences in DFS-related prediction model values were inspected between gleason score ≥7 and <7 (P=0.32), age ≥65 and age <65 (P=0.986) (Figure 8).
Ferroptosis subtypes were highly associated with pathway enrichment and immune infiltration among PCa risk groups
We examined the specific correlation of biological behaviors between PCa patients in the high- and low-risk groups, with the help of KEGG dataset to perform ssGSEA through the R package GSVA. As shown in Figure 9A, the p53 signaling pathway being the most enriched pathway in the high-risk group, and endocytosis being the most enriched pathway in the low-risk group.
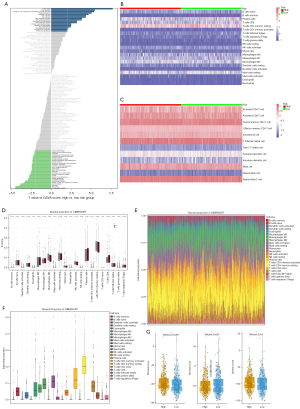
In addition, the CIBERSORT algorithm was used to visualize the relative abundance of 12 immune-infiltrating cell subsets in the dataset by generating a heat map (Figure 9B,9C). We found significant differences in ICI between the high- and low-risk groups (Figure 9D). M2-type macrophages (P=0.007) and neutrophils (P=0.024) were significantly enriched in the high-risk group, and CD4-activated memory T cells were significantly enriched in the low-risk group (P=0.017). Next, the abundance of immune infiltration of 22 immune cell subtypes in PCa tissues was assessed by a bar plot and boxplot. It was found that the relative numbers of resting memory CD4 T cells and plasma cells were the largest, while naive CD4 T cells had the lowest ratio (Figure 9E,9F). Using the Estimate algorithm, a violin plot was used to show the ratio of StromalScore, ImmuneScore, and ESTIMATEScore between the high-risk group and low-risk group, and there was an apparent difference in StromalScore between the different risk groups in the immune matrix of the tumor microenvironment (P=0.0021) (Figure 9G).
Discussion
Plenty of evidence suggests that FRGs play a role in anti-proliferation and invasion through multiple signaling pathways (13). Ferroptosis is an iron oxide-dependent selective regulator of cell death (RCD) characterized by accumulating iron-dependent extensive lipid peroxidation (14). Because of its distinct biological behavior from apoptosis, necrosis, and autophagy, ferroptosis can play a role in many biochemical processes such as anabolism and redox processes, which was also the biological process of the outcome of most malignant tumors (15). So far, there have been several studies on the role of FRGs in PCa. Liu et al. established a model with 7 FRGs and concluded that high-risk PCa patients were positively correlated with tumor mutational burden (TMB) and bicalutamide resistance (16). Lv et al. established a prediction model including 9 FRGs and calculated that a high-risk score was positively correlated with the Ki67 expression level, iron potential index, and BCR risk in PCa patients, which illustrating that FRGs are associated with prognosis in PCa patients (17). With the advancement of accurate follow-up and individualized treatment of tumors, cancer treatment has entered a new era based on genes, that is, replacing tumor types based on tumor genes or molecular profiles (18). It is crucial to avoid overdiagnosis and overtreatment of tumors (19). In this study, FRG models were established to predict OS and DFS among PCa patients, and the immune infiltration landscape was systematically analyzed in an effort to perform a transcriptomic molecular assessment.
Enrichment analysis of the differential expression of FRGs was performed. The 249 RNA-seq expression data were collected from the TCGA-PRAD and the clinical data of 500 patients. The GO results showed that the most enriched terms were certain metabolic pathways, which is consistent with the pathophysiological mechanism of ferroptosis confirmed in previous studies (20). KEGG results with higher enrichment included microRNAs in cancer, ferroptosis, arachidonic acid metabolism, and thyroid hormone synthesis. Interferon γ (IFNγ) binding to arachidonic acid (AA) induced immunogenic tumor ferroptosis as a mode of action in CD8+ T cell (CTL)-mediated tumor killing (21). Study by Mishima et al. suggested that reverse T3, a physiologically inactive form of triiodothyronine (T3), also prevents ferroptosis like other thyroid hormones (22).
Based on the difference analysis and univariate and LASSO regression analysis results of tumor and adjacent tissues, 31 FRGs associated with OS and 16 FRGs associated with DFS were obtained. Finally, the prediction models of OS and DFS were established by multivariate Cox regression analysis, which proved to be independent prognostic indexes for PCa patients.
Among ALOX12, AKR1C2, SRC, and BNIP3, and the 4 genes involved in the establishment of the OS-related prognostic model, high expression of SRC (P=0.04) was significantly correlated with poor OS in the K-M curve. ALOX12 plays a role in tumorigenesis by promoting cellular proliferation, amino acid metabolism and lipid synthesis to trigger inflammatory responses (23). the expression of ALOX12 is significantly downregulated and inhibits the proliferation of pancreatic adenocarcinoma (PAAD) cells (24), as well as related to the stage and grade of PCa (25). In prostate cells, AKR1C2 acts as a 3-ketosteroid reductase to reduce dihydrotestosterone (DHT) levels and block the activation of androgen receptor (26). Selective deletion of AKR1C2 in PCa may promote clonal expansion of tumor cells by reducing DHT metabolism and enhancing androgen-dependent cell proliferation (27). SRC is a proto-oncogenic non-receptor tyrosine kinase that plays a role in tumor cell proliferation, survival, and invasion. SRC is one of the most active kinases in phosphoproteomics of aggressive PCa (28). Chakraborty et al. demonstrated that the proto-oncogene SRC reduced primary tumor growth and metastasis by reprogramming glucose metabolism through the encoded non-receptor tyrosine kinase c-Src (29). SRC activation was found in 28% of men who developed castration-resistant PCa after androgen deprivation therapy (30). BNIP3, regarding as a member of the BH3 family, locate mitochondria and is associated with apoptosis, autophagy, or mitophagy. Elevated expression of BNIP3 in human PCa predicts poorer prognosis (31).
Among the 8 genes involved in establishing the DFS prognostic model, namely ATG16L1, AKR1C2, MIOX, ALOX12, DRD4, IDH1, RELA, and CHMP6, Hurley et al. confirmed that charged multivesicular body protein 6 (CHMP6) is ingredient of endosomal sorting complexes required for transport (ESCRT), which is required for many cellular processes involving membrane remodeling (32). Gene knockdown of CHMP6 significantly enhances tau aggregation, resulting in damage to the endolysosomal membrane, consistent with a role for the ESCRT pathway in endolysosomal membrane repair (33). In addition, mutations in CHMP6 components have been shown to be associated with degeneration of the nervous system (34). However, so far, there is no research on CHMP6 in the field of cancer. Our study shows that its high expression is significantly correlated with better DFS in PCa, which needed further exploration. DRD4 is one of the subtypes of the dopamine receptor gene in G protein-coupled receptors (GPCRs) (35), containing a variable number of 48 bp tandem repeats (VNTRs) in the third exon, and its homozygous and heterozygous forms are associated with better and worse prognosis in PCa, respectively (36). Its expression level also correlated with prostate specific antigen (PSA) levels in PCa (37). Related studies have shown that different mutation types of the metabolic enzyme isocitrate dehydrogenase 1 (IDH1) induce ferroptosis through different pathways. Pathways that inhibit ferroptosis include mutant IDH1 to produce the oncogenic metabolite D-2-hydroxyglutarate (D-2-HG) and deletion of mutant IDH1 alleles in IDH1 heterozygous tumor cells. The pathway that promotes the accumulation of lipid ROS, which in turn promotes ferroptosis, has ectopic expression of mutant IDH1 (38). High ATG16L1 expression is a poor prognostic marker in multiple cancer types, and its knockout resensitizes lung cancer cells to cancer therapy after TGF-β-induced resistance (39). However, in a clinical study involving 458 PCa patients, the results showed that high expression of ATG16L1 was associated with lower tumor aggressiveness and better prognosis (40). Therefore, it is necessary to further explore and study the correlation between ATG16L1 expression and the prognosis of PCa patients.
The prediction model was verified by clinical data such as age, Gleason score, T stage, and N stage of PCa patients. The results showed that the OS- and DFS-prediction models were correlated with the T stage score, which was higher in T3–4 than in T1–2 (P=0.0057, P=0.000697). Moreover, the N0 stage was higher than the N1 stage in the DFS prediction model (P=0.0136), with a significant difference. T stage is an important prognostic indicator for PCa patients and is positively correlated with BCR (41). As for PCa, especially in the stage of CRPC, the occurrence of lymph node metastasis is an important indicator for judging the prognosis of patients (42).
Special biological properties of the tumor cell transcriptome, such as metabolic reorganization and immune evasion, make it resistant to multiple chemotherapeutic drugs (43). This not only affects the treatment effect, but also jeopardizes the prognosis of patients (44). We evaluated the immune infiltration landscape of FRGs in PCa and attempted to comprehensively analyze the relationship between the degree of ICI and the activity of immune-related functions in the high- and low-risk groups. Through GSEA, we found that P53 gene mutations and the pentose phosphate pathway were enriched in high-risk groups of PCa. TP53 is one of the most frequently mutated suppressor genes in PCa, and its inactivation is a mid-to-late event in PCa progression, mainly associated with mCRPC (45). Strohecker et al. described a putative stimulator of autophagy, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4), that drives flux through the pentose phosphate pathway. Knockdown of PFKFB4 in PCa cells increases p62 and ROS, but surprisingly increases autophagic flux (46). Interestingly, immune genes were equally present in the high- and low-risk groups, regardless of their enrichment status, and these results are helpful for establishing prognostic risk assessment models.
In the risk score model derived from the CIBERSORT algorithm, we found that the relative numbers of resting memory CD4+ T cells and plasma cells were the largest, while the proportion of naive CD4+ T cells was the lowest, which is highly consistent with current research on ICI. Wang et al. reported that UBASH3B (STS1) is an important gene that negatively regulates T cell receptor signaling in activated T lymphocytes involved in cancer and is associated with resting CD4+ memory T cells or de novo PCa therapeutic targets (47). IL-6 produced by prostate infection with trichomonas vaginalis impacts on the tumor microenvironment by promoting PCa cell progression after M2 macrophage polarization induction (48). The number of activated CD4+ memory T cells in the high TMB group was significantly higher than that in the low TMB group, and the TMB was associated with BCR-free survival and the infiltration of activated CD4+ memory T cells in the immune microenvironment (49). The above is consistent with our results showing that M2-type macrophages were significantly enriched in the high-risk group, while CD4-activated memory T cells were highly expressed in the low-risk group. Taken together, the risk score obtained in this study is associated with the immunosuppressive microenvironment of the tumor. Finally, through the Estimate algorithm, it could be concluded that the immune matrix score of the high-risk group was higher than that of the low-risk group. PCa is considered an immunogenic tumor. A phase II clinical trial jointly conducted by the Institute of Cancer Research London and Royal Marsden Foundation Trust suggests that some patients with advanced PCa do respond, especially those with mutations in DNA repair genes that respond well to immunotherapy (50).
In this study, we established FRG-related OS and DFS-related prediction models of PCa, and for the first time proposed that the expression of CHMP6 was associated with prognosis. It may be related to the mechanism of endosomal sorting and endolysosomal membrane repair. As for ATG16L1, it has been reported that its high expression is associated with poor cancer prognosis, but previous studies have clarified that this gene can be used as an indicator of better prognosis in PCa. Therefore, analyzing and determining the clinical significance of the conduction pathway of CHMP6 and ATG16L1 in PCa is necessary as it may become a new research and therapeutic target. However, there are many shortcomings in this study. Firstly, this study is a retrospective data analysis. Without prospective clinical trials, strong evidence cannot be provided. Secondly, the data are obtained from public platform resources, such as TCGA and KEGG databases. Since no external validation was done, the accuracy was limited. There was no data from our center, and the clinicopathological data of patients in this clinical research center can be added in subsequent studies. Undoubtedly, there is still a need for a large number of validation studies in the future.
Acknowledgments
Funding: This work was supported by the National Natural Scientific Foundation of China (Grant No. 82002711) and the Beijing Municipal Administration of Hospitals’ Youth Programme (Code: QML20200105).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-454/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-454/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Brockman JA, Alanee S, Vickers AJ, et al. Nomogram Predicting Prostate Cancer-specific Mortality for Men with Biochemical Recurrence After Radical Prostatectomy. Eur Urol 2015;67:1160-7. [Crossref] [PubMed]
- Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012;149:1060-72. [Crossref] [PubMed]
- Tang R, Xu J, Zhang B, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol 2020;13:110. [Crossref] [PubMed]
- Ghoochani A, Hsu EC, Aslan M, et al. Ferroptosis Inducers Are a Novel Therapeutic Approach for Advanced Prostate Cancer. Cancer Res 2021;81:1583-94. [Crossref] [PubMed]
- Blomme A, Ford CA, Mui E, et al. 2,4-dienoyl-CoA reductase regulates lipid homeostasis in treatment-resistant prostate cancer. Nat Commun 2020;11:2508. [Crossref] [PubMed]
- Bordini J, Morisi F, Elia AR, et al. Iron Induces Cell Death and Strengthens the Efficacy of Antiandrogen Therapy in Prostate Cancer Models. Clin Cancer Res 2020;26:6387-98. [Crossref] [PubMed]
- Rui X, Shao S, Wang L, et al. Identification of recurrence marker associated with immune infiltration in prostate cancer with radical resection and build prognostic nomogram. BMC Cancer 2019;19:1179. [Crossref] [PubMed]
- Bruschini S, Ciliberto G, Mancini R. The emerging role of cancer cell plasticity and cell-cycle quiescence in immune escape. Cell Death Dis 2020;11:471. [Crossref] [PubMed]
- Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther 2020;5:166. [Crossref] [PubMed]
- Hussein MR, Al-Assiri M, Musalam AO. Phenotypic characterization of the infiltrating immune cells in normal prostate, benign nodular prostatic hyperplasia and prostatic adenocarcinoma. Exp Mol Pathol 2009;86:108-13. [Crossref] [PubMed]
- Liu G, Ma JY, Hu G, et al. Identification and validation of a novel ferroptosis-related gene model for predicting the prognosis of gastric cancer patients. PLoS One 2021;16:e0254368. [Crossref] [PubMed]
- Fang K, Li Y, Zhang Y, et al. Comprehensive analysis based in silico study of alternative bisphenols - Environmental explanation of prostate cancer progression. Toxicology 2022;465:153051. [Crossref] [PubMed]
- Lei G, Mao C, Yan Y, et al. Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell 2021;12:836-57. [Crossref] [PubMed]
- Chen X, Kang R, Kroemer G, et al. Ferroptosis in infection, inflammation, and immunity. J Exp Med 2021;218:e20210518. [Crossref] [PubMed]
- Liu H, Gao L, Xie T, et al. Identification and Validation of a Prognostic Signature for Prostate Cancer Based on Ferroptosis-Related Genes. Front Oncol 2021;11:623313. [Crossref] [PubMed]
- Lv Z, Wang J, Wang X, et al. Identifying a Ferroptosis-Related Gene Signature for Predicting Biochemical Recurrence of Prostate Cancer. Front Cell Dev Biol 2021;9:666025. [Crossref] [PubMed]
- Ginsburg GS, Phillips KA. Precision Medicine: From Science To Value. Health Aff (Millwood) 2018;37:694-701. [Crossref] [PubMed]
- Taneja SS. Re: Screening and Prostate Cancer Mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 Years of Follow-up. J Urol 2015;194:392. [Crossref] [PubMed]
- Stockwell BR, Jiang X, Gu W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol 2020;30:478-90. [Crossref] [PubMed]
- Liao P, Wang W, Wang W, et al. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 2022;40:365-378.e6. [Crossref] [PubMed]
- Mishima E, Sato E, Ito J, et al. Drugs Repurposed as Antiferroptosis Agents Suppress Organ Damage, Including AKI, by Functioning as Lipid Peroxyl Radical Scavengers. J Am Soc Nephrol 2020;31:280-96. [Crossref] [PubMed]
- Zheng Z, Li Y, Jin G, et al. The biological role of arachidonic acid 12-lipoxygenase (ALOX12) in various human diseases. Biomed Pharmacother 2020;129:110354. [Crossref] [PubMed]
- Yang J, Wei X, Hu F, et al. Development and validation of a novel 3-gene prognostic model for pancreatic adenocarcinoma based on ferroptosis-related genes. Cancer Cell Int 2022;22:21. [Crossref] [PubMed]
- Tang K, Cai Y, Joshi S, et al. Convergence of eicosanoid and integrin biology: 12-lipoxygenase seeks a partner. Mol Cancer 2015;14:111. [Crossref] [PubMed]
- Ide H, Lu Y, Noguchi T, et al. Modulation of AKR1C2 by curcumin decreases testosterone production in prostate cancer. Cancer Sci 2018;109:1230-8. [Crossref] [PubMed]
- Gorityala S, Yang S, Montano MM, et al. Simultaneous determination of dihydrotestosterone and its metabolites in mouse sera by LC-MS/MS with chemical derivatization. J Chromatogr B Analyt Technol Biomed Life Sci 2018;1090:22-35. [Crossref] [PubMed]
- Drake JM, Paull EO, Graham NA, et al. Phosphoproteome Integration Reveals Patient-Specific Networks in Prostate Cancer. Cell 2016;166:1041-54. [Crossref] [PubMed]
- Chakraborty G, Patail NK, Hirani R, et al. Attenuation of SRC Kinase Activity Augments PARP Inhibitor-mediated Synthetic Lethality in BRCA2-altered Prostate Tumors. Clin Cancer Res 2021;27:1792-806. [Crossref] [PubMed]
- Tatarov O, Mitchell TJ, Seywright M, et al. SRC family kinase activity is up-regulated in hormone-refractory prostate cancer. Clin Cancer Res 2009;15:3540-9. [Crossref] [PubMed]
- Nollet EA, Cardo-Vila M, Ganguly SS, et al. Androgen receptor-induced integrin α6β1 and Bnip3 promote survival and resistance to PI3K inhibitors in castration-resistant prostate cancer. Oncogene 2020;39:5390-404. [Crossref] [PubMed]
- Hurley JH. ESCRTs are everywhere. EMBO J 2015;34:2398-2407. [Crossref] [PubMed]
- Fan J, Pan J, Zhang X, et al. A peptide derived from the N-terminus of charged multivesicular body protein 6 (CHMP6) promotes the secretion of gene editing proteins via small extracellular vesicle production. Bioengineered 2022;13:4702-16. [Crossref] [PubMed]
- Chen JJ, Nathaniel DL, Raghavan P, et al. Compromised function of the ESCRT pathway promotes endolysosomal escape of tau seeds and propagation of tau aggregation. J Biol Chem 2019;294:18952-66. [Crossref] [PubMed]
- Lee SI, Roney MSI, Park JH, et al. Dopamine receptor antagonists induce differentiation of PC-3 human prostate cancer cell-derived cancer stem cell-like cells. Prostate 2019;79:720-31. [Crossref] [PubMed]
- Zharinov GM, Khalchitsky SE, Loktionov A, et al. The presence of polymorphisms in genes controlling neurotransmitter metabolism and disease prognosis in patients with prostate cancer: a possible link with schizophrenia. Oncotarget 2021;12:698-707. [Crossref] [PubMed]
- Akbarian F, Abolhasani M, Dadkhah F, et al. Novel Insight into Differential Gene Expression and Clinical Significance of Dopamine Receptors, COMT, and IL6 in BPH and Prostate Cancer. Curr Mol Med 2019;19:605-19. [Crossref] [PubMed]
- Wang TX, Liang JY, Zhang C, et al. The oncometabolite 2-hydroxyglutarate produced by mutant IDH1 sensitizes cells to ferroptosis. Cell Death Dis 2019;10:755. [Crossref] [PubMed]
- Xu S, Ware KE, Ding Y, et al. An Integrative Systems Biology and Experimental Approach Identifies Convergence of Epithelial Plasticity, Metabolism, and Autophagy to Promote Chemoresistance. J Clin Med 2019;8:205. [Crossref] [PubMed]
- Huang CY, Huang SP, Lin VC, et al. Genetic variants of the autophagy pathway as prognostic indicators for prostate cancer. Sci Rep 2015;5:14045. [Crossref] [PubMed]
- Horsanali MO, Eren H, Dıl E, et al. A novel prognostic risk factor for patients undergoing radical prostatectomy: Triglyseride-glucose index. Int J Clin Pract 2021;75:e13978. [Crossref] [PubMed]
- Pezzulla D, Macchia G, Cilla S, et al. Stereotactic body radiotherapy to lymph nodes in oligoprogressive castration-resistant prostate cancer patients: a post hoc analysis from two phase I clinical trials. Clin Exp Metastasis 2021;38:519-26. [Crossref] [PubMed]
- Passat T, Touchefeu Y, Gervois N, et al. Physiopathological mechanisms of immune-related adverse events induced by anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies in cancer treatment. Bull Cancer 2018;105:1033-41. [Crossref] [PubMed]
- Chang CH, Qiu J, O'Sullivan D, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015;162:1229-41. [Crossref] [PubMed]
- Teroerde M, Nientiedt C, Duensing A, et al. Revisiting the Role of p53 in Prostate Cancer. In: Bott SRJ, Ng KL, editors. Prostate Cancer (Internet). Brisbane (AU): Exon Publications, 2021:Chapter 8.
- Strohecker AM, Joshi S, Possemato R, et al. Identification of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase as a novel autophagy regulator by high content shRNA screening. Oncogene 2015;34:5662-76. [Crossref] [PubMed]
- Wang Z, Wang Y, Peng M, et al. UBASH3B Is a Novel Prognostic Biomarker and Correlated With Immune Infiltrates in Prostate Cancer. Front Oncol 2020;9:1517. [Crossref] [PubMed]
- Han IH, Song HO, Ryu JS. IL-6 produced by prostate epithelial cells stimulated with Trichomonas vaginalis promotes proliferation of prostate cancer cells by inducing M2 polarization of THP-1-derived macrophages. PLoS Negl Trop Dis 2020;14:e0008126. [Crossref] [PubMed]
- Luo C, Chen J, Chen L. Exploration of gene expression profiles and immune microenvironment between high and low tumor mutation burden groups in prostate cancer. Int Immunopharmacol 2020;86:106709. [Crossref] [PubMed]
- Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J Clin Oncol 2020;38:395-405. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


