
Risk factors of tigecycline-associated fibrinogen reduction in patients with renal transplantation: a case-control study
Introduction
Tigecycline, a first-generation glycylcycline antibiotic, was approved by the US Food and Drug Administration (FDA) in June 2005 and the European Medicines Agency in 2006 (1). It entered the Chinese market in November 2011 (2). Tigecycline is a broadspectrum antimicrobial agent with activity against Gram-positive and Gram-negative organisms via inhibition of the synthesis of protein through its action on the 30S ribosomal subunit. It plays critical roles in combating multidrug-resistant (MDR) bacteria such as vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, carbapenem-resistant Acinetobacter baumannii, and carbapenem-resistant Klebsiella pneumoniae. As previously reported, tigecycline is well tolerated (3,4) with minimal drug interactions, and no dose adjustment is needed in patients with renal impairment, making it suitable for treatment of severe infections. Tigecycline has been extensively used for complicated intra-abdominal infections, complicated skin and skin-and-soft-tissue infections and severe community-acquired pneumonia caused by bacteria sensitive to tigecycline (5). Owing to its broadspectrum antibacterial activity and low drug resistance rate, tigecycline has been widely utilized off-label for bloodstream infections, hospital-acquired pneumonia and ventilator-associated pneumonia (6,7).
Despite being approved by the FDA in 2005, drug safety has been receiving increasing clinical attention due to the insufficient information available. The standard dosing regimen is a 100-mg loading dose followed by 50 mg twice daily with intravenous administration (8). However, a regimen of high-dose tigecycline (200 mg loading dose, followed by 100 mg twice daily) has been frequently used in clinical practice to ensure its effectiveness in severe and complicated infections (9). Thus, adverse reactions might occur more frequently and severely than in clinical trials. The most commonly reported adverse events related to tigecycline are nausea, vomiting, diarrhea, and elevated alkaline phosphatase and total bilirubin levels (10). Tigecycline-induced coagulopathy has also been documented and attracted the attention of clinicians (5). Fibrinogen, a plasma glycoprotein synthesized by liver parenchymal cells, has a major role in coagulation. In addition to prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT), hypofibrinogenemia has been observed (11). When the fibrinogen level drops to 1.5 g/L, fibrinogen infusion would be needed. Once the level is lower than 1 g/L, risk of bleeding events such as gastrointestinal bleeding, pulmonary hemorrhage and cerebral hemorrhage increased, possibly resulting in aggravation of the primary disease and threatening their lives.
Recently, there have been case reports of tigecycline-associated hypofibrinogenemia and fibrinogen (FIB) reduction (12-15). Hu et al. reported that tigecycline-related hypofibrinogenemia often appeared on the 6th day of administration and was related to the baseline level of FIB before tigecycline, the maintenance dose, therapy duration and the intra-abdominal infection (2). Similar results showing that high-dose and prolonged tigecycline therapy are risk factors of tigecycline-associated hypofibrinogenemia have been reported (16). Zhang et al. observed that regardless of the requirement for dialysis, renal failure was a risk factor for tigecycline-induced hypofibrinogenemia (17). In addition, elderly patients were more prone to developing hypofibrinogenemia after tigecycline administration, as reported previously (18). In clinical practice, patients with immunosuppression are more susceptible to bacterial infections and tend to require broadspectrum antimicrobial agents. Thus, immunosuppressed patients account for a large proportion of patients using tigecycline. As far as we know, data of tigecycline-associated hypofibrinogenemia in immunosuppressed patients have not been reported.
Therefore, we conducted a single-center and a case-control study to explore the tigecycline-associated FIB reduction in patients with renal transplantation. We analyzed the clinical features and risk factors in comparison with baseline FIB level to inform clinicians managing immunosuppressed patients receiving tigecycline to ensure safe and effective therapy. We present the following article in accordance with the STROBE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-522/rc).
Methods
Patients
A single-center and a case-control study of patients was carried out at the First Affiliated Hospital, School of Medicine, Zhejiang University, which is a university-affiliated tertiary care and teaching hospital with 2,500 beds and over 100,000 discharged patients per year. From January, 2017 to January, 2020, patients who underwent renal transplantation and were treated with tigecycline during hospitalization were enrolled. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (No. 2018-032), and individual consent for this retrospective analysis was waived.
Inclusion criteria were: (I) tigecycline therapy >2 days, and (II) FIB level >2 g/L before tigecycline use. Exclusion criteria were: (I) previous or concurrent transplantation of an organ other than a kidney, (II) incomplete record of coagulation function, (III) FIB administration during treatment, and (IV) incomplete clinical record. Patient enrolment is shown in Figure 1.
Data collection and definitions
The clinical electronic medical records were reviewed, and epidemiological, clinical, demographic and laboratory data were collected for all enrolled patients. A self-designed and standard data collection form was utilized to record basic information (sex, age, height, body weight), and the primary disease for renal transplantation. Among the possible associated factors, the laboratory results (levels of transaminase, albumin and FIB) and reason for tigecycline use were collected at initial tigecycline administration. Renal transplantation type was obtained from the surgical records. Immune-induction regimen was collected immediately after renal transplantation. Tigecycline use status were collected after discontinuation of tigecycline. Recovery of renal function was recorded in the 7 days post-operation. All data were confirmed by three independent researchers. The relationship between FIB reduction and tigecycline exposure was assessed by Naranjo’s Probability Scale in adverse drug reaction evaluation. To describe the clinical features of tigecycline-associated FIB reduction, the time of the occurrence of hypofibrinogenemia was recorded and evaluated.
For the definitions of renal function recovery after renal transplantation, delayed graft function (DGF) referred to acute kidney injury and dialysis intervention in the first week of renal transplantation (19). Slow graft function (SGF) represented serum creatinine level >2.5 mg/dL within 5 days. Abnormal transaminases referred to levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) exceeding three times the upper limit of the normal level. Empirical therapy was therapy with tigecycline deemed necessary by two independent senior physicians based on the patient’s clinical symptoms. Based on the previous research hypofibrinogenemia was defined as plasma FIB <2.0 g/L (2). The extent of the decrease in FIB was calculated by comparison with the baseline level of FIB before tigecycline administration. FIBRO was defined as FIB reduction >50%, and FIBRB was FIB reduction <50%.
Immunosuppression regimen
The recipients underwent immune-induction therapy with antithymocyte globulin (ATG) or interleukin-2 receptor antagonist (basiliximab). Patients received standard triple-drug immunosuppression regimen after renal transplantation. The triple therapy comprised corticosteroid (prednisone), calcineurin inhibitor (tacrolimus or cyclosporine), and an antiproliferative agent (mycophenolate mofetil or enteric-coated mycophenolate sodium). The trough concentration of tacrolimus was maintained at 4–10 ng/mL, and that of cyclosporine was controlled at 150–250 ng/mL within the 12 months postoperatively. When acute rejection was diagnosed, a 3–5-day course of methylprednisolone (6–10 mg/kg/day) was added to the antirejection therapy regimen. If first-line treatment failed, the recipients were treated with ATG (1.0 mg/kg/day for 5 days) or plasma exchange therapy for humoral rejection.
Statistical analysis
SPSS (ver. 22.0) was utilized for statistical analysis. Continuous variables are displayed as mean [standard deviation (SD)] (normal distribution) or as median [interquartile range (IQR)] (non-normal distribution). Student’s t-test or the Mann-Whitney U-test, as appropriate, was used to compare the baseline of the two groups of subjects. Categorical variables are presented as frequency (percentage) and assessed using the Pearson χ2 or Fisher’s exact test (cell size <5).
A chi-squared test was used for categorical variables. Univariate and multivariate analyses were conducted by logistic regression models to identify independent risk factors of tigecycline-associated FIBRO. Laboratory test indicators (levels of transaminase, albumin and FIB), type of renal transplantation, renal function recovery after surgery and tigecycline therapy status (manufacturer, therapy duration, and total dose) were investigated. The factors showing a significant association in the univariate logistic regression analysis [95% confidence interval (CI): does not include 1] were entered into the multivariable logistic regression analysis. A P value <0.05 was considered to indicate statistical significance.
Results
Demographics of the study population
During the study period, 1,008 renal transplantations were performed at the Kidney Disease Center. Of them, 123 patients were treated with tigecycline for >2 days and after exclusions, 120 patients were enrolled. Their demographic data are presented in Table 1. The mean age was 44.32±12.14 (range, 8–70) years, and the mean body mass index (BMI) was 20.92±3.12. Unidentified kidney diseases (n=60, 50%) and IgA glomerulonephritis (n=51, 41.5%) were the most common primary kidney diseases.
Table 1
| Characteristics | All patients |
|---|---|
| Age, years | |
| Mean (SD) | 44.32 (12.14) |
| Range | 8–70 |
| Sex | |
| Male | 74 (61.67%) |
| Female | 46 (38.33%) |
| BMI (kg/m2) | 20.92 (3.12) |
| Primary disease | |
| Unidentified diseases | 60 (50%) |
| Glomerulonephritis (IgA) | 51 (42.5%) |
| Polycystic kidney | 6 (5%) |
| Diabetic nephropathy | 1 (0.83%) |
| Other kidney diseases | 2 (1.67) |
Data are presented as n (%) and mean (SD) unless specified otherwise. SD, standard deviation; BMI, body mass index.
Clinical features and outcomes of tigecycline-associated hypofibrinogenemia
In our study, 7 patients (5.83%) had a FIB reduction <30%, 26 patients (21.67%) had a FIB reduction of 30–50%, and 79 patients (65.83%) had a reduction of >50%. Based on our definition of plasma FIB <2.0 g/L, the clinical features of tigecycline-associated hypofibrinogenemia were analyzed first. Most of the patients (n=114, 95.00%) developed hypofibrinogenemia from 1 to 11 days {median (25–75th percentile) of 3 [2–4] days} after initiation of tigecycline use (Figure 2). Prompt intervention and timely FIB administration prevented serious events such as bleeding. Nevertheless, compared with the patients with normal FIB levels, those with hyperfibrinogenemia had an increased length of hospital stay with significant increases in medical and total costs (Table 2).
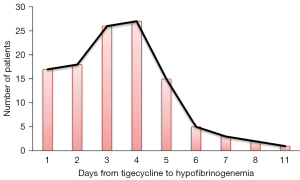
Table 2
| Variable | Hypofibrinogenaemia group (n=114) | Normal group (n=6) | P |
|---|---|---|---|
| Length of hospital stay (days) | 16.60±8.27 | 11.83±2.48 | <0.001 |
| Total cost (¥) | 140,962.14±53,736.76 | 115,373.4±15,219.72 | 0.014 |
| Medicine cost (¥) | 74,249.18±31,499.48 | 55,439.8±7,125.67 | <0.001 |
Data are presented as mean ± SD. SD, standard deviation.
The limited sample size for the normal group resulted in insufficient statistical power to analyze the risk factors of tigecycline-associated hypofibrinogenemia.
Demographics of the FIBRO and FIBRB groups
The sample sizes of the FIBRO and FIBRB groups were 79 and 41, respectively. Males accounted for 61.67% (n=74) of the patients (58.23% in FIBRO and 68.29% in FIBRB group). The mean age of patients in FIBRO and FIBRB groups was 45.04±12.18 (range, 11–70) and 42.93 ±12.10 (range, 8–63) years, respectively (Table 3).
Table 3
| Characteristics | FIBRO (n=79) | FIBRB (n=41) |
|---|---|---|
| Age, years | ||
| Mean (SD) | 45.04 (12.18) | 42.93 (12.10) |
| Range | 11–70 | 8–63 |
| Sex | ||
| Male | 46 (58.23%) | 28 (68.29%) |
| Female | 33 (41.77%) | 13 (31.71%) |
| BMI (kg/m2) | 21.27 (2.99) | 20.28 (3.28) |
| Primary disease | ||
| Unidentified diseases | 37 (46.84%) | 23 (56.10%) |
| Glomerulonephritis (IgA) | 37 (46.84%) | 14 (34.15%) |
| Polycystic kidney | 3 (3.80%) | 3 (7.32%) |
| Diabetic nephropathy | 1 (1.27%) | 0 |
| Other kidney diseases | 1 (1.27%) | 1 (2.44%) |
Data are presented as n (%) and mean (SD) unless specified otherwise. FIB, fibrinogen; FIBRO, the extent of FIB reduction over 50%; FIBRB, the extent of FIB reduction below 50%; SD, standard deviation; BMI, body mass index.
Clinical characteristics and univariate logistic analysis for risks factors for FIBRO
The majority of the study population (n=105, 87.5%) had normal levels of transaminases before tigecycline administration: albumin and FIB were 36.02±4.38 and 3.47±1.01 g/L, respectively. Most of the patients (n=116, 96.67%) received a kidney from donation after cardiac death (DCD) or after brain death (DBD). Basiliximab (n=79, 65.83%) or ATG (n=34, 28.33%) was used for immune-induction therapy. The renal function of most patients recovered immediately after surgery (n=94, 78.33%), and the remaining patients showed SGF or DGF. Of the 120 patients, half (43.33%) were administered empirical tigecycline therapy, and the others were dosed according definite microbiological culture and drug susceptibility results in vitro. The patients received 7.54±3.58 days (range, 2–26 days) of tigecycline therapy with total dose of 1.001±0.479 g (Table 4).
Table 4
| Variable | FIBRO (n=79) | FIBRB (n=41) | OR (95% CI) | P |
|---|---|---|---|---|
| Laboratory results | ||||
| Albumin level (g/L) | 35.96±4.36 | 36.14±4.48 | 1.008 (0.977–1.041) | 0.606 |
| FIB level before tigecycline use | 3.78±0.98 | 2.89±0.80 | 3.285 (1.880–5.740) | <0.001 |
| Abnormal transaminase level | 10 (12.66%) | 5 (12.20%) | 1.043 (0.332–3.285) | 0.942 |
| Renal transplantation type | ||||
| Living-related transplantation | 2 (2.53%) | 2 (4.88%) | Used as reference | |
| DCD | 63 (79.75%) | 31 (75.61%) | 2.032 (0.273–15.116) | 0.489 |
| DBD | 14 (17.72%) | 8 (19.51%) | 1.750 (0.205–14.931) | 0.609 |
| Immune-induction regimen | ||||
| Basiliximab | 53 (67.09%) | 26 (63.41%) | Used as reference | |
| ATG | 20 (25.32%) | 14 (34.15%) | 0.701 (0.306–1.605) | 0.400 |
| Others | 6 (7.59%) | 1 (2.44%) | 2.943 (0.337–25.738) | 0.329 |
| Recovery of renal function | ||||
| Immediate | 62 (78.48%) | 32 (78.05%) | Used as reference | |
| SGF/DGF | 17 (21.52%) | 9 (21.95%) | 0.975 (0.391–2.431) | 0.957 |
| Reason for tigecycline use | ||||
| Targeted therapy | 42 (53.16%) | 26 (63.41%) | Used as reference | |
| Empirical therapy | 37 (46.84%) | 15 (36.59%) | 1.527 (0.704–3.311) | 0.284 |
| Tigecycline use status | ||||
| Manufacturer | ||||
| Patheon Italia S.p.A. | 46 (58.23%) | 20 (48.78%) | Used as reference | |
| Hisun Pharmaceutical Co., Ltd. | 33 (41.77%) | 21 (51.22%) | 0.683 (0.320–1.458) | 0.325 |
| Therapy duration (days) | 8.11±3.56 | 6.44±3.38 | 1.173 (1.030–1.336) | 0.016 |
| Total dose (g) | 1.096±0.504 | 0.819±0.368 | 5.879 (1.777–19.452) | 0.004 |
Data are presented as n (%) and mean ± SD unless specified otherwise. FIB, fibrinogen; FIBRO, the extent of FIB reduction over 50%; FIBRB, the extent of FIB reduction below 50%; DCD, donation after cardiac death; DBD, donation after brain death; ATG, antithymocyte globulin; SGF, slow graft function; DGF, delayed graft function; SD, standard deviation.
Risk factors for FIBRO
Univariable and multivariable logistic regression models were used to explore the independent the risk factors for FIBRO (Table 4). The univariate logistic analyses demonstrated that the baseline FIB level before tigecycline use (3.78±0.98 g/L in FIBRO group vs. 2.89±0.80 in FIBRB group, P<0.001) was significantly related with FIBRO. Tigecycline therapy duration (8.11±3.56 days in FIBRO group vs. 6.44±3.38 days in FIBRB group, P=0.016) and the total tigecycline dose [odds ratio (OR): 4.930, 95% confidence interval (CI): 1.433–16.959, P=0.004] were also significantly associated with FIBRO. However, FIBRO was not related to the transaminase or albumin level, type of renal transplantation, renal function recovery after surgery or tigecycline manufacturer. Because the total tigecycline dose probably correlated with therapy duration, we conducted a correlation analysis. The results showed a Pearson correlation coefficient between total dose and duration of 0.915, indicating a strong correlation between the two parameters. Therefore, the variables showing a significant relation to FIBRO with P<0.1 in the univariate analyses were included in the multivariate analysis: baseline FIB level before tigecycline use and the total tigecycline dose. As shown in Table 5, the FIB level before tigecycline use (OR: 3.225, 95% CI: 1.801–5.772, P<0.001) and the total tigecycline dose (OR: 4.930, 95% CI: 1.433–16.959, P=0.011) were significantly related to FIBRO.
Table 5
| Variable | Multivariate analysis | P | |
|---|---|---|---|
| OR | 95% CI | ||
| FIB level before tigecycline use | 3.225 | 1.801–5.772 | <0.001 |
| Total dose | 4.930 | 1.433–16.959 | 0.011 |
FIB, fibrinogen.
Discussion
Infection caused by MDR bacteria has become a dominant challenge in human health (20). It has been predicted that bacterial infections could result in 10 million deaths each year by 2050 (21). Due to its broadspectrum antibacterial activity and good tolerance, tigecycline is often administered for severe infections. Except for off-label use, it is frequently used with high doses and prolonged therapy to ensure efficacy (22,23). According to package insert provided by the manufacturer, gastrointestinal symptoms are the most common adverse reactions, whereas adverse reactions involving the blood and lymphatic system, such as aPTT and PT prolongation and an increased INR are <2%. The adverse events of FIB reduction and hypofibrinogenemia are not listed. With widespread use of tigecycline, severe cases and retrospective studies have been reported (12,24-26) and did not include immunosuppressed patients, who account for a large proportion of the patients administered tigecycline. Because data of tigecycline-associated hypofibrinogenemia and FIB reduction in immunosuppressed patients have not been reported, we limited our study population to patients undergoing renal transplantation and tigecycline therapy.
Fibrinogen, a 340-kDa plasma glycoprotein, is synthesized by liver parenchymal cells with half time of 3–4 days and has a major role in coagulation (27). The normal FIB level in blood varies between 2 and 4 g/L. A reduction in the FIB level commonly occurs in patients with coexisting chronic inherited diseases, acquired hepatic dysfunction and severe malnutrition. In our study, the relationship between FIB reduction and tigecycline exposure was assessed by Naranjo’s Probability Scale in adverse drug reaction evaluation. All of the cases of FIB reduction in our study were most likely related to tigecycline.
In this study, tigecycline-associated hypofibrinogenemia mainly appeared at 3 days after tigecycline administration, which was earlier than in the reported literature. Routsi et al. found that high doses of tigecycline led to a gradual reduction in fibrinogen levels after 14 days of administration, and prolonged INR and aPTT, based on data from 45 patients in an intensive care unit (28). A retrospective study by Leng et al demonstrated that tigecycline treatment could lead to statistically significant FIB reduction, and aPTT and PT prolongation 4 days after the initial dosage (11). In another study, hyperfibrinogenemia developed at a median of 6 [4–8] days after tigecycline treatment (2). We speculate that reason for these differences can be attributed to the specific enrolled populations. The frequency of tigecycline-associated hypofibrinogenemia in our study population was 95.00%, significantly higher than reported in the previous literature. Renal failure is reported to be a risk factor for tigecycline-induced hypofibrinogenemia (17). Our study population comprised patients with renal transplantation, so the adverse reaction of FIB reduction might occur more frequently and earlier.
Another result of our study was that the FIB level before tigecycline use and the total tigecycline dose were significantly associated with FIBRO, consistent with the previous research. Zhang et al. reported hypofibrinogenemia in 80% of 20 enrolled patients with severe infections and treated with tigecycline, and hypofibrinogenemia was proportional to the dose administered (29). Campany-Herrero et al. found that high-dose tigecycline treatment and treatment duration >4 weeks were risk factors for a reduction in fibrinogen plasma concentration >1.7 g/L (16). As previously reported, tigecycline-associated hypofibrinogenemia is reversible and the FIB level normalizes within days of tigecycline discontinuation (30).
Our study has some limitations. Firstly, it was a retrospective study and selection bias might exist during the patient enrolment procedure. Secondly, due to the concerns of coagulation disorder, FIB was administered when the FIB level significantly decreased. Therefore, the correlation between the laboratory indicator and clinical outcomes such as the bleeding-related adverse events, and recovery of FIB level were not investigated. Finally, tigecycline therapy was divided into targeted therapy and empiric therapy. The specific pathogenic bacteria or the infection site were not analyzed. Further randomized controlled trials with larger populations or animal studies investigating the potential mechanism of tigecycline-associated FIB reduction are urgently needed. Despite these limitations, the risk factors of tigecycline-associated FIB reduction >50% in renal transplantation patients were identified and clinicians should be aware of FIB reduction and bleeding risk when administrating immunosuppressed patients with tigecycline.
Conclusions
FIB reduction frequently occurs in renal transplantation patients receiving tigecycline therapy and mainly occurs at 3 days after tigecycline administration, resulting in increased length of hospital stay, as well as costs for the patient. FIBRO was significantly related with the FIB level before tigecycline use and the total tigecycline dose, but not to the transaminase or albumin level, type of renal transplantation, renal function recovery after surgery or tigecycline manufacturer. We propose that in addition to the FIB level, coagulation-related indicators such as PT, APTT, and INR should be closely monitored during tigecycline treatment to avoiding life-threatening coagulation and bleeding events.
Acknowledgments
Funding: The research was supported by National Natural Science Foundation of China (Nos. 81971981 and 82003660), the Nature Science Foundation of Zhejiang province (Nos. LQ20H300003, LYY21H300004, and LYY18H310003), and Zhejiang Pharmaceutical Association Hospital Pharmacy Special Scientific Research Funding Project (Nos. 2019ZYY02, 2014ZYY04, and 2019ZYYYG01).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-522/rc
Data Sharing Statement: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-522/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-522/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (No. 2018-032), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leng B, Yan G, Wang C, et al. Dose optimisation based on pharmacokinetic/pharmacodynamic target of tigecycline. J Glob Antimicrob Resist 2021;25:315-22. [Crossref] [PubMed]
- Hu J, Xiao YH, Zheng Y, et al. Clinical characteristics and risk factors of tigecycline-associated hypofibrinogenaemia in critically ill patients. Eur J Clin Pharmacol 2020;76:913-22. [Crossref] [PubMed]
- Perutelli A, Tascini C, Domenici L, et al. Safety and efficacy of tigecycline in complicated and uncomplicated pelvic inflammatory disease. Eur Rev Med Pharmacol Sci 2018;22:3595-601. [PubMed]
- Guirao X, Sánchez García M, Bassetti M, et al. Safety and tolerability of tigecycline for the treatment of complicated skin and soft-tissue and intra-abdominal infections: an analysis based on five European observational studies. J Antimicrob Chemother 2013;68:ii37-44. [Crossref] [PubMed]
- Cui N, Cai H, Li Z, et al. Tigecycline-induced coagulopathy: a literature review. Int J Clin Pharm 2019;41:1408-13. [Crossref] [PubMed]
- Ben Mabrouk A, Ben Brahim H, Kooli I, et al. Off label uses of tigecycline. Ann Pharm Fr 2021;79:244-54. [Crossref] [PubMed]
- Chen Z, Shi X. Adverse events of high-dose tigecycline in the treatment of ventilator-associated pneumonia due to multidrug-resistant pathogens. Medicine (Baltimore) 2018;97:e12467. [Crossref] [PubMed]
- Rubinstein E, Vaughan D. Tigecycline: a novel glycylcycline. Drugs 2005;65:1317-36. [Crossref] [PubMed]
- Zha L, Pan L, Guo J, et al. Effectiveness and Safety of High Dose Tigecycline for the Treatment of Severe Infections: A Systematic Review and Meta-Analysis. Adv Ther 2020;37:1049-64. [Crossref] [PubMed]
- Kadoyama K, Sakaeda T, Tamon A, et al. Adverse event profile of tigecycline: data mining of the public version of the U.S. Food and Drug Administration adverse event reporting system. Biol Pharm Bull 2012;35:967-70. [Crossref] [PubMed]
- Leng B, Xue YC, Zhang W, et al. A Retrospective Analysis of the Effect of Tigecycline on Coagulation Function. Chem Pharm Bull (Tokyo) 2019;67:258-64. [Crossref] [PubMed]
- Fan Q, Huang W, Weng Y, et al. Hypofibrinogenemia induced by high-dose tigecycline-case report and review of literature. Medicine (Baltimore) 2020;99:e22638. [Crossref] [PubMed]
- Wu PC, Wu CC. Tigecycline-associated hypofibrinogenemia: A case report and review of the literature. IDCases 2018;11:56-7. [Crossref] [PubMed]
- Yılmaz Duran F, Yıldırım H, Şen EM. A Lesser Known Side Effect of Tigecycline: Hypofibrinogenemia. Turk J Haematol 2018;35:83-4. [Crossref] [PubMed]
- Pieringer H, Schmekal B, Biesenbach G, et al. Severe coagulation disorder with hypofibrinogenemia associated with the use of tigecycline. Ann Hematol 2010;89:1063-4. [Crossref] [PubMed]
- Campany-Herrero D, Larrosa-Garcia M, Lalueza-Broto P, et al. Tigecycline-associated hypofibrinogenemia in a real-world setting. Int J Clin Pharm 2020;42:1184-9. [Crossref] [PubMed]
- Zhang Q, Wang J, Liu H, et al. Risk factors for tigecycline-induced hypofibrinogenaemia. J Clin Pharm Ther 2020;45:1434-41. [Crossref] [PubMed]
- Liu J, Yan Y, Zhang F. Risk Factors for Tigecycline-Associated Hypofibrinogenemia. Ther Clin Risk Manag 2021;17:325-32. [Crossref] [PubMed]
- Bahl D, Haddad Z, Datoo A, et al. Delayed graft function in kidney transplantation. Curr Opin Organ Transplant 2019;24:82-6. [Crossref] [PubMed]
- Bassetti M, Righi E, Carnelutti A, et al. Multidrug-resistant Klebsiella pneumoniae: challenges for treatment, prevention and infection control. Expert Rev Anti Infect Ther 2018;16:749-61. [Crossref] [PubMed]
- Giono-Cerezo S, Santos-Preciado JI, Morfín-Otero MDR, et al. Antimicrobial resistance. Its importance and efforts to control it. Gac Med Mex 2020;156:171-8. [Crossref] [PubMed]
- Han H, Qin W, Zheng Y, et al. High-Dose versus Standard-Dose Tigecycline Treatment of Secondary Bloodstream Infections Caused by Extensively Drug-Resistant Acinetobacter baumannii: An Observational Cohort Study. Infect Drug Resist 2021;14:3837-48. [Crossref] [PubMed]
- Cunha BA, Baron J, Cunha CB. Once daily high dose tigecycline - pharmacokinetic/pharmacodynamic based dosing for optimal clinical effectiveness: dosing matters, revisited. Expert Rev Anti Infect Ther 2017;15:257-67. [Crossref] [PubMed]
- Treml B, Rajsic S, Hell T, et al. Progression of Fibrinogen Decrease during High Dose Tigecycline Therapy in Critically Ill Patients: A Retrospective Analysis. J Clin Med 2021;10:4702. [Crossref] [PubMed]
- Hakeam HA, Al Duhailib Z, Salahuddin N, et al. Impact of tigecycline versus imipenem-cilastatin on fibrinogen levels following cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): a randomized-controlled study. J Chemother 2018;30:224-32. [Crossref] [PubMed]
- Sabanis N, Paschou E, Gavriilaki E, et al. Hypofibrinogenemia induced by tigecycline: a potentially life-threatening coagulation disorder. Infect Dis (Lond) 2015;47:743-6. [Crossref] [PubMed]
- Ahuja CS, Fehlings M. Concise Review: Bridging the Gap: Novel Neuroregenerative and Neuroprotective Strategies in Spinal Cord Injury. Stem Cells Transl Med 2016;5:914-24. [Crossref] [PubMed]
- Routsi C, Kokkoris S, Douka E, et al. High-dose tigecycline-associated alterations in coagulation parameters in critically ill patients with severe infections. Int J Antimicrob Agents 2015;45:90-3. [Crossref] [PubMed]
- Zhang Q, Zhou S, Zhou J. Tigecycline treatment causes a decrease in fibrinogen levels. Antimicrob Agents Chemother 2015;59:1650-5. [Crossref] [PubMed]
- Balfousias T, Apostolopoulos AP, Angelis S, et al. Spontaneous Knee Hemarthrosis Due to Hypofibrinogenemia Following Tigecycline Treatment for Periprosthetic Joint Infection. Cureus 2019;11:e5883. [Crossref] [PubMed]



