
A narrative review of peripheral blood parameters for urothelial carcinoma treated with systemic antitumor drugs
Introduction
Bladder cancer is the tenth most common malignancy worldwide (1,2) and occurs in the renal pelvis, ureters, and urethra (2). The majority of bladder cancers are the urothelial carcinoma (UC) subtype. Patients with advanced UC (aUC), including those with unresectable, locally advanced and metastatic disease, mainly receive systemic chemotherapy with palliative intervention. For the past 30 years, the gold standard of first-line therapy has been cisplatin-based chemotherapy (2). Prior to the arrival of immune checkpoint inhibitors (ICIs) in the US, the 5-year survival rate for all bladder cancer stages is 77% but that of patients with aUC is approximately 5% (2,3). Currently, ICIs, such as atezolizumab, nivolumab, pembrolizumab, avelumab, and durvalumab, have received US Food and Drug administration approval for the treatment of urothelial carcinoma (2). Consequently, the approval of various drugs is expected to improve the prognosis of patients with aUC. In addition, the choice of treatment is becoming more important than ever before. Easy-to-measure and useful parameters predicting the effectiveness of such drugs and the prognosis of patients are in demand in clinical practice. However, appropriate methods of predicting a prognosis have not yet been established.
An association between inflammation and tumor progression has been known for decades (4). There is increasing evidence that pro-inflammatory cells in the tumor microenvironment actively contribute to tumor progression (4). Furthermore, the elevated levels of pro-inflammatory cytokines that accompany tumorigenesis often lead to cachexia, which is the process of involuntary loss of muscle and adipose tissue. Markers of cachexia including high blood cell counts and increased levels of C-reactive protein (CRP) in the serum of patients. Given the close association between systemic inflammation and cachexia, there is intense basic and clinical research into the optimal combination of blood-based markers that should be used to monitor these pathologies in cancer patients. Indeed, assessment of such markers in combination with other clinical data such as body mass can be used to predict prognosis. In this review, we discuss various parameters for patients with aUC who were treated with antitumor drugs. In particular, we deal with parameters obtained from blood tests that are related to inflammation and the assessment of nutrition. We present this article in accordance with the Narrative Review reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-805/rc).
Methods
A comprehensive literature search was conducted using PubMed and Google Scholar databases up to October 2022. Search terms included “nutrition”, “urothelial”, “bladder”, “prognosis”, “chemotherapy”, “immunotherapy”, “urology”, “prognostic biomarker”, or “predictive biomarker”. A summary of the search strategy is shown in Table 1. We also manually searched articles related to this topic. The studies were reviewed by three authors (TN, TN, and YS) to assess whether they were appropriate.
Table 1
| Items | Specification |
|---|---|
| Date of search | 2022.10.31 |
| Databases and other sources searched | PubMed, Google Scholar |
| Search terms used | Search terms included “nutrition”, “urothelial”, “bladder”, “prognosis”, “chemotherapy”, “immunotherapy”, “urology”, “predictive biomarker”, or “prognostic biomarker” |
| Time frame | 1950.01–2022.10 |
| Inclusion and exclusion criteria | Inclusion criteria |
| • Focus on urothelial carcinoma and biomarkers | |
| • Article written in English | |
| • Peer-reviewed articles including original article and review | |
| Exclusion criteria | |
| • None | |
| Selection process | First author and corresponding author and third author selected articles, and all co-authors approved reference lists |
Results
Inflammatory parameters and nutritional parameters
Peripheral blood parameters for cancer treatment are categorized as either predictive or prognostic. Predictive parameters are factors that predict the efficacy or side effects of a drug or other treatment. They are used for selecting cases for whom such drugs would be useful. In comparison, prognostic parameters are used for determining prognosis, with or without treatment. They are indicators that are used to identify the likelihood of the future appearance, recurrence, or development of clinical signs. In clinical practice, the development of biomarkers combined with body composition and blood tests has been attempted. Such biomarkers reflect the balance of factors related to cancer progression and suppression.
Cachexia and parameters
In general, cachexia is seen in half of patients with cancer at diagnosis and is responsible for approximately 20% of cancer deaths (5,6). The body weight loss observed in patients with cancer is categorized into two types: cancer-related and cancer-induced body weight loss. Cancer-related body weight loss results from a disorder of ingestion, digestion, and absorption. Cancer-induced body weight loss is a result of metabolic disorders based on host–tumor interactions, such as cytokines from inflammation or protein degradation inducers released from cancer. This weight loss appears irreversible and early screening of patients is important for the early intervention of supportive nutritional therapy.
Cancer cells induce systemic inflammation and this leads to body weight loss via a loss of appetite and the decomposition of skeletal muscle and fatty tissue (7,8). Appetite loss is one of the characteristic complaints of patients with cancer cachexia and is related to ghrelin, an endogenous hormone secreted from the stomach. Ghrelin has various effects: stimulation of appetite, inhibition of muscle protein degradation, promotion of muscle protein synthesis, inhibition of apoptosis, among others (9). Cancer and the biological response to cancer causes an increase in proteolysis-inducing factors and inflammatory cytokines, such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α. These mediators induce abnormal skeletal muscle metabolism and muscle atrophy (8). Cancer cells also promote the decomposition of fatty tissue. Tumor-derived lipid mobilizing factor accelerates lipolysis, and IL-6 and parathyroid hormone–related protein alter adipocytes to form brown adipocytes (10). Brown adipocytes lead to increasing thermogenesis and inefficient energy consumption via the expression of uncoupling protein 1 (10). Thus, systemic inflammation and malnutrition progress as cancer progresses.
Screening for inflammation and malnutrition in patients is important for the decision-making process in cancer treatment. Early diagnosis and intervention are necessary since the treatment of cachexia in its advanced stages is difficult. Multimodal treatments, such as drugs, nutrition, exercise therapies, and psychosocial interventions, are needed to treat cachexia (11,12). In addition to being necessary for early diagnosis, assessing inflammation and malnutrition using blood-based markers could be used to evaluate a change in the condition of patients over time. Patients with cancer are often older and may have difficulty in continuing their treatment due to the severity of side effects induced by some of the available therapeutic agents. The treatment of cachexia should be tailored to a patient’s condition and lifestyle to ensure continuity of care, which is why screening for inflammation and malnutrition is useful. We summarized the correlation of component and prognostic biomarker in Figure 1, and listed the characteristics of blood test–based parameters in Table 2.
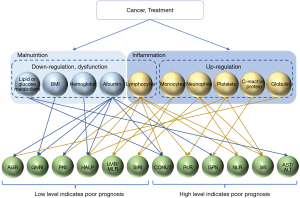
Table 2
| Items | Specimen | Formula |
|---|---|---|
| NLR | CBC | Neutrophil/Lymphocyte |
| PLR | CBC | Platelet/Lymphocyte |
| MLR | CBC | Monocyte/Lymphocyte |
| SII | CBC | Neutrophil × Platelet/Lymphocyte |
| SIRI | CBC | Neutrophil × Monocyte/Lymphocyte |
| HALP score | CBC, SC | Hemoglobin × Alb × Lymphocyte/Platelet |
| De Ritis ratio | SC | Aspartate aminotransferase/Alanine aminotransferase |
| GPS/mGPS | SC | GPS score |
| 0: CRP ≤1.0 mg/dL and Alb ≥3.5 g/dL | ||
| 1: CRP >1.0 mg/dL or Alb <3.5 g/dL | ||
| 2: CRP >1.0 mg/dL and Alb <3.5 g/dL | ||
| Modified GPS score | ||
| 0: CRP ≤0.5 mg/dL and Alb ≥3.5 g/dL | ||
| 1: CRP >0.5 mg/dL or Alb <3.5 g/dL | ||
| 2: CRP >0.5 mg/dL and Alb <3.5 g/dL | ||
| GNRI | SC, height, BW | 14.89 × Alb (g/dL) + 41.7 × BW (kg) / ideal BW (kg) |
| PNI | CBC, SC | 10 × Alb (g/dL) + 0.005 × Lymphocyte |
| CONUT | SC | Alb score: 0 (3.5≤ Alb), 2 (3≤ Alb <3.5), 4 (2.5≤ Alb <3), 6 (Alb <2.5) |
| TLC score: 0 (1,800≤ TLC), 1 (1,200≤ TLC <1,800, 2 (800≤ TLC <1,200), 3 (TLC <800) | ||
| T-cho score: 0 (180≤ T-cho), 1 (140≤ T-cho <180), 2 (100≤ T-cho <140), 3 (T-cho <100) | ||
| CONUT is the sum of Alb score, TLC score, and T-cho score | ||
| 0–1 normal, 2–4 mild, 5–8 moderate, 9–12 severe | ||
| AGR | SC | Alb / (total protein - Alb) |
AGR, albumin to globulin ratio; Alb, albumin; BW, body weight; CBC, complete blood count; CRP, c-reactive protein; GNRI, geriatric nutritional risk index; GPS, Glasgow prognostic score; HALP, hemoglobin, albumin, lymphocyte, and platelet; mGPS, modified GPS; MLR, monocyte-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SC, serum chemistries; SII, systemic immune–inflammation index; SIRI, systemic inflammatory response index; TLC, total peripheral lymphocyte count; T-cho, total cholesterol.
Blood test items reflecting cancer progression and suppression
Hemoglobin
Hemoglobin is produced in the bone marrow and constitutes a major component of red blood cells. More than 30% of patients with cancer have cancer-related anemia at diagnosis (13). Such anemia is a result of anticancer therapy, hemorrhage from cancer, bone marrow infiltration, malnutrition, and chronic inflammation (14,15). Inflammatory cytokines are associated with erythropoietin production, erythroid progenitors, and reticuloendothelial iron release (16). In a prospective study, cancer stage correlated with the prevalence and severity of anemia (15). A reduction in the hemoglobin level reflects chronic inflammation and malnutrition in cancer patients.
Neutrophils
At an early stage of inflammation, neutrophils migrate and try to eliminate foreign objects. Hence, infiltration of neutrophils expresses the inflammation of tumor (17,18). In addition, neutrophils play an important role in tumor progression and metastasis by producing cytokines and chemokines (19,20). Moreover, suppression of lymphocyte function by activated neutrophils could be mediated by the secretion of myeloperoxidase, further contributing to cancer progression by dampening the immune response (21).
Monocytes
Monocytes are produced in the bone marrow and circulate in blood vessels, bone marrow, and spleen. They play a role in homeostasis, inflammation, and antimicrobial immune defense (22). In tumor tissues, monocytes differentiate to macrophages and induce inflammation (23). They promote tumor proliferation, increased angiogenesis, and inhibit antitumor immunity (23).
Lymphocytes
Lymphocytes, such as natural killer T cells and cytotoxic T lymphocytes, contribute to cytotoxic immunity and reflect anti-tumor immunity (24,25). They inhibit tumor cell proliferation and metastasis (17,25). Therefore, the number of lymphocytes indicates the state of cancer immunity.
Platelets
The differentiation of megakaryocytes to platelets is activated by secretion of cytokines and chemokines by tumor cells (26). Platelets promote tumor growth, angiogenesis, and metastasis by interacting with tumor cells (27). Moreover, thrombosis mediated by platelets is strongly associated with inflammation (28). In clinical practice, paraneoplastic thrombocytosis reflects chronic inflammation and its presence defines a specific subtype of malignancies that originate from different tissues (29). Thus, an increased platelet count reflects tumor progression.
CRP
CRP is an acute phase proteins (APP) produced by hepatocytes during the inflammatory response. Its production and secretion are stimulated by the cytokines IL-6, TNF, and IL-1 (30,31). Although not a cancer-specific marker, CRP levels correlate with those of IL-6 and can therefore be used as an indicator of inflammation in cancer patients. Two main hypotheses exist regarding CRP elevation in patients with cancer. First, tumor cells may increase the levels of CRP indirectly during the induction of inflammation. Alternatively, tumor cells may themselves secrete factors that lead to increased CRP levels. The value of CRP alone as a prognostic biomarker for patients with UC has been assessed in multiple clinical trials. For aUC patients, it has been reported that elevated CRP was associated with both worse PFS and OS (32). As CRP measurement is simple and inexpensive, it can serve as a non-specific biomarker of response for initial treatment.
Albumin
Circulating serum albumin (Alb) is an indicator of general health and nutritional status, and its levels are reduced during malnutrition and inflammation (30,33), and malnutrition itself slightly affects hypoalbuminemia (34). Independently of malnutrition, inflammation leads to increased capillary permeability, which decreases the half-life of albumin, thereby inducing hypoalbuminemia (35).
Globulin
The difference between the amount of serum albumin relative to the total amount of serum protein is known as the ‘gamma gap’ or ‘GG’. In dialysis patients and in patients dying from pulmonary complications, the GG has been used as an independent predictor of mortality (36,37). The globulin family includes α-globulin, β-globulin, and γ-globulins; the latter class includes immunoglobulins such as IgG and IgM. The globulins play an important role in immunity and chronic inflammation, and their levels reflect a cumulative exposure of different cytokines.
The aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio
AST and ALT are critical regulators of cellular metabolism and cancer cell turnover, and have potential utility as blood biomarkers. De Ritis et al. were the first to show that the AST/ALT ratio (De Ritis ratio) was a useful indicator of etiology in patients with acute hepatitis (38). Since then, multiple studies have shown that the AST/ALT ratio is a prognostic indicator in cancer patients. Since an increased ratio is associated with a higher rate of anerobic glycolysis, which is a hallmark of UC, it could be used as a prognostic indicator in this malignancy. Indeed, an elevated De Ritis ratio was significantly associated with worse prognosis and higher mortality in patients with UC who underwent radical cystectomy (39-42).
Parameters obtained from peripheral blood tests and body composition
Neutrophil-to-lymphocyte ratio
The ratio of neutrophil to lymphocyte counts (NLR) was first reported as a prognostic marker in patients treated in an intensive care unit (43). The NLR is a formula in which the number of neutrophils (which reflects cancer progression) is set as the numerator and the number of lymphocytes (which reflects cancer suppression) is set as the denominator. Thus, the NLR is a well-balanced marker that reflects tumor progression.
In the setting of aUC patients treated with cisplatin-based chemotherapy, an elevated NLR was associated with poor overall survival (OS) (44-48), a poorer response to chemotherapy and poor prognosis (44-46). A greater change in the NLR was a marker of poor prognosis in patients with aUC (49); it was also reported in advanced or metastatic patients with upper urinary tract urothelial cancer (UTUC) (50). Furthermore, NLR was described as a prognostic marker before pembrolizumab was available. After setting the cut-off of NLR as 2.9–4.0, a high NLR at pretreatment was a predictive marker of OS in aUC patients (51-54). A change in NLR between pre- and posttreatments was also described as a marker of aUC (51,55,56). In addition to a high NLR, the presence of metastatic lesions was also shown to be a risk factor (52,57,58). Moreover, a high NLR after the start of pembrolizumab treatment and changes in the NLR are also negatively associated with OS (54,57-59). In a meta-analysis, the risk factors for aUC despite pembrolizumab treatment were: poor performance status, liver metastasis, a higher pretreatment NLR and/or CRP (60). Similarly, when treating with atezolizumab, NLR can be used as a predictive marker of OS (61). Furthermore, a high NLR was associated with a poor OR for patients with aUC treated with pembrolizumab (62).
Platelet-to-lymphocyte ratio (PLR)
The PLR is also another biomarker derived from the peripheral blood. The PLR is a formula in which the number of platelets (which reflects cancer progression) is set as the numerator and the number of lymphocytes (which reflects cancer suppression) is set as the denominator. As with NLR, the PLR is a reasonable nutritional index that combines inflammatory and immune indices. There are no published data regarding a potential correlation between the PLR and the prognosis of aUC patients treated with cisplatin-based chemotherapy. A pretreatment PLR (cut-off ≥173.73) was associated with worse OS in aUC patients treated with pembrolizumab (54,62). A high PLR was associated with a poor initial tumor response to treatment with pembrolizumab (62).
Lymphocyte-to-monocyte ratio (LMR)/monocyte-to-lymphocyte ratio (MLR)
The LMR is a formula in which the number of monocytes (which reflects cancer progression) is set as the numerator and the number of lymphocytes (which reflects cancer suppression) is set as the denominator. The MLR, a logarithm of LMR, is also used. Whether LMR or MLR can be used as predictive or prognostic factors in aUC patients treated with cisplatin-based chemotherapy remains unclear. The high MLR before pembrolizumab in aUC was associated with poor OS following pembrolizumab treatment (54).
Systemic immune–inflammation index
A formula for the systemic immune–inflammation index (SII) consists of the number of neutrophils multiplied by the number of platelets (which reflects cancer progression) and is set as the numerator, and the number of lymphocytes (which reflects cancer suppression) that is set as the denominator. In short, the SII is represented as NLR × platelets or PLR × neutrophils. In a study of a Caucasian population, a prolonged high SII at 6 weeks was a predictor of poor progression-free survival (PFS) and poor OS of aUC patients treated with cisplatin-based chemotherapy (63). As for aUC patients treated with ICIs, the combination of SII with programmed death ligand 1 (PD-L1), with or without lactate dehydrogenase may represent an easy-to-assess, cheap, and readily available prognostic tool (64). Moreover, the combination of SII with PD-L1, with or without LDH, can be used as a predictor of PFS (64).
Systemic inflammatory response index (SIRI)
A formula for the SIRI consists of the number of monocytes multiplied by the number of neutrophils (which reflects cancer progression) and is set as the numerator, and the number of lymphocytes (which reflects cancer suppression) which is set as the denominator. In short, the SIRI is represented by MLR × neutrophils or NLR × monocytes. Whether SIRI can be used as a predictive or prognostic factor in aUC patients treated with cisplatin-based chemotherapy remains unclear. The SIRI before pembrolizumab therapy in advanced UC correlated with OS after pembrolizumab treatment (54). However, it is unclear whether SIRI is a predictive factor for aUC patients treated with ICIs.
Hemoglobin, albumin, lymphocyte, and platelet (HALP) score
The HALP score is calculated according to the following formula: hemoglobin (g/L) × Alb (g/L) × lymphocytes (/L)/platelets (/L). There are no reports regarding the association between a HALP score and aUC patients treated with cisplatin-based chemotherapy. In a cohort of patients treated with pembrolizumab, a HALP score <30.05 was associated with worse OS (62). Whether the HALP score is predictive of outcome in aUC patients treated with ICIs is unclear.
Glasgow prognostic score (GPS)/modified GPS
The GPS is a combined index of CRP and Alb levels (65). It was reported as a prognostic predictive factor for non-small cell lung cancer in 2003 (65). Patients with elevated CRP (cut-off: 1.0 mg/dL) and hypoalbuminemia (cut-off; 3.5 mg/dL) are scored as GPS 2; patients with elevated CRP or hypoalbuminemia are scored as GPS 1; and patients without elevated CRP and hypoalbuminemia are scored as GPS 0. A modified GPS (mGPS) with a different cut-off for CRP level (0.5 mg/dL) has also been reported (66). In a cohort of patients with advanced bladder cancer treated with gemcitabine plus cisplatin, the GPS correlated with poor OS (67). In a setting of cisplatin-based chemotherapy, a modified (m)GPS was predictive of OS (68). In cohorts of patients treated with ICIs (atezolizumab, pembrolizumab, and nivolumab), the mGPS was associated with a poor prognosis (69). A higher mGPS, especially mGPS 2, reflected a poor prognosis and poor PFS of aUC patients treated with pembrolizumab as second-line therapy (68).
Nutritional risk index (NRI)/Geriatric nutritional risk index (GNRI)
In the past, the NRI was used to assess malnutrition (70). However, it was difficult to identify the usual body weight of elderly people. To resolve this problem, the NRI was modified by replacing usual body weight with ideal body weight; this was named the GNRI (71). It is calculated from three values: the Alb concentration, ideal body weight, and actual body weight. Moreover, height was estimated based on knee height because it was difficult to measure the height of hospitalized patients such as those who are bedridden (71). It is also beneficial to adopt the GNRI for elderly patients. However, knee height is not often used. In several reports, ideal body weight (defined based on height and a BMI of 22, which is considered clinically healthy) is also used to calculate GNRI (72,73). Few reports exist regarding the GNRI as prognostic parameter for patients with aUC. In the first-line setting of cisplatin-based chemotherapy, low GNRI and visceral metastasis were reported as predictive parameters (74). Similarly in patients treated with pembrolizumab, a low GNRI (cut-off 92) was predictive of a poor prognosis (75). The incidence of fatigue as an adverse event was high in the low-GNRI group (76). With the inclusion of pembrolizumab, gemcitabine, and docetaxel therapy, a low GNRI (cut-off 92) was indicative of a worse prognosis (76).
Prognostic nutritional index (PNI)
The first report of the PNI was as a predictor of perioperative complications (77). It was calculated based on measurements of Alb, triceps subcutaneous fat thickness, serum transferrin, and delayed cutaneous hypersensitivity reaction. It was modified to a simple form using Alb and lymphocyte counts. The PNI is calculated using the following formula: 0.005 × total lymphocyte count + 10 × Alb. For aUC patients treated with cisplatin- or carboplatin-based chemotherapy, the PNI (cut-off 40) was an independent risk factor for a poor prognosis (78). Moreover, a low PNI was associated with visceral metastases, leukocytosis, and anemia (78). In the setting of pembrolizumab treatment of aUC patients, the PNI was shown to be an independent predictor of PFS (77). The PNI (cut-off 40–45) was also described as a predictive factor for OS (53,79).
Controlling Nutritional Status score
The Controlling Nutritional Status score (CONUT) is a combined score calculated from total peripheral lymphocyte count (TLC), total cholesterol concentration, and serum albumin concentration (80). It has been reported as a prognostic factor for esophageal cancer and colorectal cancer (81). Alb score is calculated as 0 (3.5≤ Alb), 2 (3≤ Alb <3.5), 4 (2.5≤ Alb <3), and 6 (Alb <2.5). The TLC score is calculated as 0 (1,800≤ TLC), 1 (1,200≤ TLC <1,800, 2 (800≤ TLC <1,200), and 3 (TLC <800). The total cholesterol (T-cho) score is calculated as 0 (180≤ T-cho), 1 (140≤ T-cho <180), 2 (100≤ T-cho <140), and 3 (T-cho <100) respectively. The CONUT score is the sum of Alb, TLC, and T-cho scores, and is classified into 0–1 (normal), 2–4 (mild), 5–8 (moderate), and 9–12 (severe). A CONUT score of 4 or greater is associated with poor OS in urothelial carcinoma (82).
Albumin-globulin ratio
The albumin-to-globulin ratio (AGR) is the ratio of serum Alb to non-albumin proteins. Malnutrition and inflammatory cytokines can inhibit the production of Alb, resulting in low serum Alb concentration, which could stimulate cell proliferation and weaken immune defense mechanisms. Globulin contains several immune-related proteins, such as complement components, fibrinogen and serum amyloid A, which are involved in regulating immunity and inflammation. However, using Alb and/or globulin alone produces unstable results, and its measurement is susceptible to interference by external confounders. AGR has a higher predictive value when combined with measurements of globulin and serum albumin compared to measurements of either of these parameters in isolation (83). Although the exact cut-off value for AGR has not been determined, aUC patients with a low AGR and who were treated with pembrolizumab had shorter PFS and OS than patients with a high AGR (84).
Conclusions
Various biomarkers are related to systemic inflammation and malnutrition. They are useful in clinical practice because they are easily obtained from routine blood tests. However, the definitive cut-off for each biomarker is still unclear. Using biomarkers from various studies as reference points is useful in deciding on treatment strategies for aUC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-805/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-22-805/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-805/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Bellmunt J, Valderrama BP, Puente J, et al. Recent therapeutic advances in urothelial carcinoma: A paradigm shift in disease management. Crit Rev Oncol Hematol 2022;174:103683. [Crossref] [PubMed]
- Saginala K, Barsouk A, Aluru JS, et al. Epidemiology of Bladder Cancer. Med Sci (Basel) 2020;8:15. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [Crossref] [PubMed]
- von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle 2010;1:1-5. [Crossref] [PubMed]
- von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle 2016;7:507-9. [Crossref] [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Amitani M, Asakawa A, Amitani H, et al. Control of food intake and muscle wasting in cachexia. Int J Biochem Cell Biol 2013;45:2179-85. [Crossref] [PubMed]
- Molfino A, Formiconi A, Rossi Fanelli F, et al. Ghrelin: from discovery to cancer cachexia therapy. Curr Opin Clin Nutr Metab Care 2014;17:471-6. [Crossref] [PubMed]
- Argilés JM, Busquets S, Stemmler B, et al. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014;14:754-62. [Crossref] [PubMed]
- De Waele E, Mattens S, Honoré PM, et al. Nutrition therapy in cachectic cancer patients. The Tight Caloric Control (TiCaCo) pilot trial. Appetite 2015;91:298-301. [Crossref] [PubMed]
- Katakami N, Uchino J, Yokoyama T, et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 2018;124:606-16. [Crossref] [PubMed]
- Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 2004;40:2293-306. [Crossref] [PubMed]
- Madeddu C, Gramignano G, Astara G, et al. Pathogenesis and Treatment Options of Cancer Related Anemia: Perspective for a Targeted Mechanism-Based Approach. Front Physiol 2018;9:1294. [Crossref] [PubMed]
- Macciò A, Madeddu C, Gramignano G, et al. The role of inflammation, iron, and nutritional status in cancer-related anemia: results of a large, prospective, observational study. Haematologica 2015;100:124-32. [Crossref] [PubMed]
- Means RT Jr, Krantz SB. Progress in understanding the pathogenesis of the anemia of chronic disease. Blood 1992;80:1639-47. [Crossref] [PubMed]
- Brigati C, Noonan DM, Albini A, et al. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis 2002;19:247-58. [Crossref] [PubMed]
- Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105:93-103. [Crossref] [PubMed]
- Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016;16:431-46. [Crossref] [PubMed]
- Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res 2011;71:2411-6. [Crossref] [PubMed]
- el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol 1987;139:2406-13. [Crossref] [PubMed]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011;11:762-74. [Crossref] [PubMed]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39-51. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature 2001;411:380-4. [Crossref] [PubMed]
- Haemmerle M, Stone RL, Menter DG, et al. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018;33:965-83. [Crossref] [PubMed]
- Jain S, Harris J, Ware J. Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol 2010;30:2362-7. [Crossref] [PubMed]
- Wagner DD. New links between inflammation and thrombosis. Arterioscler Thromb Vasc Biol 2005;25:1321-4. [Crossref] [PubMed]
- Lin RJ, Afshar-Kharghan V, Schafer AI. Paraneoplastic thrombocytosis: the secrets of tumor self-promotion. Blood 2014;124:184-7. [Crossref] [PubMed]
- Saito K, Kihara K. Role of C-reactive protein in urological cancers: a useful biomarker for predicting outcomes. Int J Urol 2013;20:161-71. [Crossref] [PubMed]
- Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem 2004;279:48487-90. [Crossref] [PubMed]
- O'Brian D, Prunty M, Hill A, et al. The Role of C-Reactive Protein in Kidney, Bladder, and Prostate Cancers. Front Immunol 2021;12:721989. [Crossref] [PubMed]
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [Crossref] [PubMed]
- McMillan DC, Watson WS, O'Gorman P, et al. Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer 2001;39:210-3. [Crossref] [PubMed]
- Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: Pathogenesis and Clinical Significance. JPEN J Parenter Enteral Nutr 2019;43:181-93. [Crossref] [PubMed]
- Juraschek SP, Moliterno AR, Checkley W, et al. The Gamma Gap and All-Cause Mortality. PLoS One 2015;10:e0143494. [Crossref] [PubMed]
- Avramovski PJ, Petlichkovski A, Avramovska M, et al. The Gamma Gap Predicts All-Cause Mortality in Chronic Dialysis Patients. Indian J Nephrol 2021;31:212-7. [Crossref] [PubMed]
- De Ritis F, Coltorti M, Giusti G. An enzymatic test for the diagnosis of viral hepatitis; the transaminase serum activities. Clin Chim Acta 1957;2:70-4. [Crossref] [PubMed]
- Ghahari M, Salari A, Ghafoori Yazdi M, et al. Association Between Preoperative De Ritis (AST/ALT) Ratio and Oncological Outcomes Following Radical Cystectomy in Patients With Urothelial Bladder Cancer. Clin Genitourin Cancer 2022;20:e89-93. [Crossref] [PubMed]
- Ha YS, Kim SW, Chun SY, et al. Association between De Ritis ratio (aspartate aminotransferase/alanine aminotransferase) and oncological outcomes in bladder cancer patients after radical cystectomy. BMC Urol 2019;19:10. [Crossref] [PubMed]
- Eriksson V, Holmkvist O, Huge Y, et al. A Retrospective Analysis of the De Ritis Ratio in Muscle Invasive Bladder Cancer, with Focus on Tumor Response and Long-Term Survival in Patients Receiving Neoadjuvant Chemotherapy and in Chemo Naïve Cystectomy Patients-A Study of a Clinical Multicentre Database. J Pers Med 2022;12:1769. [Crossref] [PubMed]
- Yuk HD, Jeong CW, Kwak C, et al. De Ritis Ratio (Aspartate Transaminase/Alanine Transaminase) as a Significant Prognostic Factor in Patients Undergoing Radical Cystectomy with Bladder Urothelial Carcinoma: A Propensity Score-Matched Study. Dis Markers 2019;2019:6702964. [Crossref] [PubMed]
- Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 2001;102:5-14. [PubMed]
- Tan YG, Eu EWC, Huang HH, et al. High neutrophil-to-lymphocyte ratio predicts worse overall survival in patients with advanced/metastatic urothelial bladder cancer. Int J Urol 2018;25:232-8. [Crossref] [PubMed]
- Auvray M, Elaidi R, Ozguroglu M, et al. Prognostic Value of Baseline Neutrophil-to-Lymphocyte Ratio in Metastatic Urothelial Carcinoma Patients Treated With First-line Chemotherapy: A Large Multicenter Study. Clin Genitourin Cancer 2017;15:e469-76. [Crossref] [PubMed]
- Yip SM, Kaiser J, Li H, et al. Real-world Outcomes in Advanced Urothelial Cancer and the Role of Neutrophil to Lymphocyte Ratio. Clin Genitourin Cancer 2018;16:e637-44. [Crossref] [PubMed]
- Taguchi S, Nakagawa T, Matsumoto A, et al. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of survival in patients with metastatic urothelial carcinoma: A multi-institutional study. Int J Urol 2015;22:638-43. [Crossref] [PubMed]
- Su YL, Hsieh MC, Chiang PH, et al. Novel Inflammation-Based Prognostic Score for Predicting Survival in Patients with Metastatic Urothelial Carcinoma. PLoS One 2017;12:e0169657. [Crossref] [PubMed]
- Rossi L, Santoni M, Crabb SJ, et al. High neutrophil-to-lymphocyte ratio persistent during first-line chemotherapy predicts poor clinical outcome in patients with advanced urothelial cancer. Ann Surg Oncol 2015;22:1377-84. [Crossref] [PubMed]
- Hashimoto M, Fujita K, Nakayama T, et al. Higher neutrophil-to-lymphocyte ratio after the first cycle of the first-line chemotherapy is associated with poor cancer specific survival of upper urinary tract carcinoma patients. Transl Androl Urol 2021;10:2838-47. [Crossref] [PubMed]
- Yamamoto Y, Yatsuda J, Shimokawa M, et al. Prognostic value of pre-treatment risk stratification and post-treatment neutrophil/lymphocyte ratio change for pembrolizumab in patients with advanced urothelial carcinoma. Int J Clin Oncol 2021;26:169-77. [Crossref] [PubMed]
- Ito K, Kobayashi T, Kojima T, et al. Pembrolizumab for treating advanced urothelial carcinoma in patients with impaired performance status: Analysis of a Japanese nationwide cohort. Cancer Med 2021;10:3188-96. [Crossref] [PubMed]
- Shimizu T, Miyake M, Hori S, et al. Clinical Impact of Sarcopenia and Inflammatory/Nutritional Markers in Patients with Unresectable Metastatic Urothelial Carcinoma Treated with Pembrolizumab. Diagnostics (Basel) 2020;10:310. [Crossref] [PubMed]
- Kadono Y, Kawaguchi S, Nohara T, et al. Blood Cell Count Biomarkers Predicting Efficacy of Pembrolizumab as Second-line Therapy for Advanced Urothelial Carcinoma. Anticancer Res 2021;41:1599-606. [Crossref] [PubMed]
- Ogihara K, Kikuchi E, Shigeta K, et al. The pretreatment neutrophil-to-lymphocyte ratio is a novel biomarker for predicting clinical responses to pembrolizumab in platinum-resistant metastatic urothelial carcinoma patients. Urol Oncol 2020;38:602.e1-602.e10. [Crossref] [PubMed]
- Fukata S, Mizutani K, Yamamoto S, et al. Sarcopenia and the rate of change of the neutrophil/lymphocyte ratio as predictors of pembrolizumab efficacy in advanced urothelial carcinoma. Anticancer Drugs 2022;33:459-66. [Crossref] [PubMed]
- Tomioka-Inagawa R, Nakane K, Enomoto T, et al. The Impact of Neutrophil-to-Lymphocyte Ratio after Two Courses of Pembrolizumab for Oncological Outcomes in Patients with Metastatic Urothelial Carcinoma. Biomedicines 2022;10:1609. [Crossref] [PubMed]
- Uchimoto T, Komura K, Fukuokaya W, et al. Risk Classification for Overall Survival by the Neutrophil-Lymphocyte Ratio and the Number of Metastatic Sites in Patients Treated with Pembrolizumab-A Multicenter Collaborative Study in Japan. Cancers (Basel) 2021;13:3554. [Crossref] [PubMed]
- Tamura D, Jinnouchi N, Abe M, et al. Prognostic outcomes and safety in patients treated with pembrolizumab for advanced urothelial carcinoma: experience in real-world clinical practice. Int J Clin Oncol 2020;25:899-905. [Crossref] [PubMed]
- Yanagisawa T, Mori K, Katayama S, et al. Pretreatment clinical and hematologic prognostic factors of metastatic urothelial carcinoma treated with pembrolizumab: a systematic review and meta-analysis. Int J Clin Oncol 2022;27:59-71. [Crossref] [PubMed]
- Tural D, Ölmez ÖF, Sümbül AT, et al. Prognostic factors in patients with metastatic urothelial carcinoma who have treated with Atezolizumab. Int J Clin Oncol 2021;26:1506-13. [Crossref] [PubMed]
- Kurashina R, Ando K, Inoue M, et al. Platelet-to-Lymphocyte Ratio Predicts the Efficacy of Pembrolizumab in Patients With Urothelial Carcinoma. Anticancer Res 2022;42:1131-6. [Crossref] [PubMed]
- Palacka P, Slopovsky J, Obertova J, et al. Survival Prediction by Baseline Systemic Immune-inflammation Index (SII) and its Changes During First-line Platinum-based Treatment in a Caucasian Population of Patients With Metastatic Urothelial Carcinoma (MUC). Anticancer Res 2021;41:5749-59. [Crossref] [PubMed]
- Fornarini G, Rebuzzi SE, Banna GL, et al. Immune-inflammatory biomarkers as prognostic factors for immunotherapy in pretreated advanced urinary tract cancer patients: an analysis of the Italian SAUL cohort. ESMO Open 2021;6:100118. [Crossref] [PubMed]
- Forrest LM, McMillan DC, McArdle CS, et al. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 2003;89:1028-30. [Crossref] [PubMed]
- Toiyama Y, Miki C, Inoue Y, et al. Evaluation of an inflammation-based prognostic score for the identification of patients requiring postoperative adjuvant chemotherapy for stage II colorectal cancer. Exp Ther Med 2011;2:95-101. [Crossref] [PubMed]
- Hwang EC, Hwang IS, Yu HS, et al. Utility of inflammation-based prognostic scoring in patients given systemic chemotherapy first-line for advanced inoperable bladder cancer. Jpn J Clin Oncol 2012;42:955-60. [Crossref] [PubMed]
- Nagai T, Naiki T, Isobe T, et al. Modified Glasgow Prognostic Score 2 as a Prognostic Marker in Patients With Metastatic Urothelial Carcinoma. In Vivo 2021;35:2793-800. [Crossref] [PubMed]
- Brown JT, Liu Y, Shabto JM, et al. Baseline Modified Glasgow Prognostic Score Associated with Survival in Metastatic Urothelial Carcinoma Treated with Immune Checkpoint Inhibitors. Oncologist 2021;26:397-405. [Crossref] [PubMed]
- Buzby GP, Williford WO, Peterson OL, et al. A randomized clinical trial of total parenteral nutrition in malnourished surgical patients: the rationale and impact of previous clinical trials and pilot study on protocol design. Am J Clin Nutr 1988;47:357-65. [Crossref] [PubMed]
- Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr 2005;82:777-83. [Crossref] [PubMed]
- Matsunaga T, Saito H, Osaki T, et al. Impact of geriatric nutritional risk index on outcomes after gastrectomy in elderly patients with gastric cancer: a retrospective multicenter study in Japan. BMC Cancer 2022;22:540. [Crossref] [PubMed]
- Ide S, Okugawa Y, Omura Y, et al. Geriatric nutritional risk index predicts cancer prognosis in patients with local advanced rectal cancer undergoing chemoradiotherapy followed by curative surgery. World J Surg Oncol 2021;19:34. [Crossref] [PubMed]
- Naiki T, Nagai T, Sugiyama Y, et al. First Report of Oncological Outcome and Prognostic Analysis in a First-Line Setting of Short Hydration Gemcitabine and Cisplatin Chemotherapy for Patients with Metastatic Urothelial Carcinoma. Oncology 2021;99:622-31. [Crossref] [PubMed]
- Etani T, Naiki T, Sugiyama Y, et al. Low Geriatric Nutritional Risk Index as a Poor Prognostic Marker for Second-Line Pembrolizumab Treatment in Patients with Metastatic Urothelial Carcinoma: A Retrospective Multicenter Analysis. Oncology 2020;98:876-83. [Crossref] [PubMed]
- Isobe T, Naiki T, Sugiyama Y, et al. Chronological transition in outcome of second-line treatment in patients with metastatic urothelial cancer after pembrolizumab approval: a multicenter retrospective analysis. Int J Clin Oncol 2022;27:165-74. [Crossref] [PubMed]
- Buzby GP, Mullen JL, Matthews DC, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg 1980;139:160-7. [Crossref] [PubMed]
- Hsieh MC, Rau KM, Chiang PH, et al. Impact of Prognostic Nutritional Index on Overall Survival for Patients with Metastatic Urothelial Carcinoma. J Cancer 2018;9:2466-71. [Crossref] [PubMed]
- Ishiyama Y, Kondo T, Nemoto Y, et al. Predictive Impact of Prognostic Nutritional Index on Pembrolizumab for Metastatic Urothelial Carcinoma Resistant to Platinum-based Chemotherapy. Anticancer Res 2021;41:1607-14. [Crossref] [PubMed]
- Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005;20:38-45. [PubMed]
- Toyokawa T, Kubo N, Tamura T, et al. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: results from a retrospective study. BMC Cancer 2016;16:722. [Crossref] [PubMed]
- Une M, Ito M, Suzuki H, et al. Controlling Nutritional Status (CONUT) Score and Sarcopenia as Mutually Independent Prognostic Biomarkers in Advanced Urothelial Carcinoma. Cancers (Basel) 2022;14:5075. [Crossref] [PubMed]
- Xia Z, Fu X, Li J, et al. Prognostic value of pretreatment serum albumin-globulin ratio in urothelial carcinoma: A systematic review and meta-analysis. Front Oncol 2022;12:992118. [Crossref] [PubMed]
- Taguchi S, Kawai T, Nakagawa T, et al. Prognostic significance of the albumin-to-globulin ratio for advanced urothelial carcinoma treated with pembrolizumab: a multicenter retrospective study. Sci Rep 2021;11:15623. [Crossref] [PubMed]


