
Effects of clean intermittent catheterization and transurethral indwelling catheterization on the management of urinary retention after gynecological surgery: a systematic review and meta-analysis
Highlight box
Key findings
• Clean intermittent catheterization can lower the incidence of urinary tract infections, reduce residual urine volume, shorten the duration of catheter maintenance, and improve bladder function recovery.
What is known and what is new?
• Based on previous study results, there is no consistent conclusion regarding the choice between indwelling catheterization and clean intermittent catheterization after gynecological surgery.
• Clean intermittent catheterization can lower the incidence of urinary tract infections, reduce residual urine volume, shorten the duration of catheter days, and improve bladder function recovery in patients with urinary retention after gynecological surgery.
What is the implication, and what should change now?
• Clean intermittent catheterization may be more effective in patients undergoing radical cervical cancer resection.
Introduction
Urinary retention is a common bladder functional disorder after gynecological surgery, with an estimated prevalence of 2.5–24% (1). Patients with urinary retention usually have difficulties passing urine and voluntarily emptying the bladder within 6–8 hours after surgery, and this condition is typically accompanied by bladder pain and distension. The incidence of urinary retention varies among different types of gynecological surgery: after pelvic surgery, its incidence is estimated to be between 2.5% and 43% (2); following cesarean section, its incidence is between 7.4% and 16.7% (3); after vaginal delivery surgery, its incidence is 1.7–17.9% (4,5); and after radical cervical cancer surgery, its incidence is as high as 8–80% (6).
Bladder voiding dysfunction is a common complication in patients undergoing cervical cancer surgery, the main cause of which is a surgical injury to the bladder detrusor, urethral sphincter, or pelvic autonomic nerves (7,8). The surgery-induced anatomical changes lead to defects in the supporting structures of the pelvic floor, and the absence of support for the urethra and bladder neck allows urine to accumulate in the bladder, making it difficult to urinate. Overextension of the bladder due to a postoperative pelvic hematoma, inflammation, and adhesions is also responsible for short-term bladder paralysis. Moreover, long-term postoperative urinary catheterization and poor psychological status also affect the physiological filling and emptying ability of the bladder and increase the risk of urinary retention. Compared with conventional hysterectomy, modified surgical methods in recent years, such as nerve-sparing radical surgery, can reduce the risk of postoperative bladder dysfunction (9). Unfortunately, the evidence for this is low quality, and further large-scale, high-quality randomized controlled trials (RCTs) are required to verify this conclusion (10).
Urinary retention is responsible for multiple adverse outcomes. Apart from bladder compression injury caused by dysuria, patients may also suffer from urinary tract infections (UTIs) and urinary incontinence caused by the continuous accumulation of urine, as well as other complications, such as hydronephrosis, secondary bacteremia, and septicemia in serious cases (11), posing an enormous burden on patients both physically and psychologically. Bladder drainage is currently applied as the primary approach in the management of urinary retention, including various measures of transurethral indwelling catheterization, clean intermittent catheterization, and suprapubic catheterization.
Indwelling catheterization is the most frequently applied means for both short- and long-term catheterization. This measure involves catheter insertion into the bladder passing through the urethra, and the catheter is left in place to allow urine drainage. Patients with indwelling catheters may need to replace the urine drainage bag and catheter regularly. As UTI is the most common complication of indwelling catheterization, it is critical to avoid unnecessary catheterization and remove the catheter as soon as possible. However, whether this conclusion applies to high-risk patients is uncertain (12).
Clean intermittent catheterization is a technique that applies a clean or reusable catheter to empty the bladder at regular intervals, and the catheter is removed immediately after voiding the bladder, which can be implemented either by a healthcare provider or by the patient (or caregiver). The clean intermittent catheterization technique has been confirmed to be safe and effective in patients suffering from neurogenic lower urinary tract dysfunction, which improves renal and upper urinary tract status, reduces vesicoureteral reflux, and improves urinary incontinence (13). Bakke et al. (14) pointed out that catheter-associated UTIs were associated with a lower frequency of catheterization in a questionnaire survey and microbiological study of patients with clean intermittent catheterization. A network meta-analysis by Han et al. explored the incidence of UTIs from different catheterization protocols and their findings indicated that the clean intermittent catheterization technique is a favorable option with a satisfactory effect for both short- and long-term catheterization, as compared to indwelling catheterization and suprapubic catheterization (15).
In actual clinical practice, indwelling catheterization is the most commonly used postoperative bladder drainage method since it is applied earlier in clinical treatment and is simple and familiar for most medical workers (16). However, indwelling catheterization is found to be associated with a higher incidence of UTI. Therefore, choosing the optimal treatment plan for patients is challenging. Although some guidelines recommended that doctors and nursing staff should use intermittent catheterization to reduce a series of complications such as UTI (17), there is a lack of reliable evidence, which hampers the development of appropriate bladder management guidelines. Despite the small number of studies that have been previously performed to evaluate the clinical efficacy of different catheterization methods, the review of Han et al. (15) assessed the effectiveness of the short-term application of indwelling catheterization, clean intermittent catheterization, and suprapubic catheterization in patients with urinary retention in all conditions, focusing only on UTIs as the outcome indicator. Similarly, the review by Li et al. (18) also highlighted the incidence of UTIs. Based on the previous study results, there is no consistent conclusion regarding the choice between indwelling catheterization and clean intermittent catheterization after gynecological surgery.
Therefore, this study conducted the current systematic review to evaluate the efficacy and safety of clean intermittent catheterization and transurethral indwelling catheter catheterization in patients with urinary retention after gynecological surgery, aiming to provide more powerful evidence for the clinical management of this condition. We present this article in accordance with the PRISMA reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-23-220/rc) (19).
Methods
Database search
The search strategy employed in this study adopted the combination of subject words and free words for retrieval from seven medical databases: PubMed, Web of Science, Cochrane Library, Embase, China National Knowledge Infrastructure (CNKI), Wanfang Data, and the Chinese Scientific Journal Database (VIP). The search language is limited to English and Chinese, and there is no restriction on the region. The search time is from the inception of the databases to November 2022. The specific search strategy is shown in Appendix 1.
Inclusion and exclusion criteria
Inclusion criteria: (I) participants: female patients aged ≥18 years old complaining of urinary retention after gynecological surgery; Currently, no standard definition of urinary retention has been established in clinical practice. The commonly used criteria are inability to urinate spontaneously after surgery, residual urine volume after self-urination exceeding 100 to 200 mL or exceeding 1/2 to 1/3 of the total bladder volume (2). (II) intervention: clean intermittent catheterization; (III) control: routine transurethral indwelling catheterization; (IV) outcomes: Incidence of UTIs, catheter days, and residual urine volume; (V) study design: RCTs.
Exclusion criteria: We excluded reviews, guidelines, abstracts, conference papers, opinions, letters, and case reports because these types of publications usually lacked certain quantitative information.
Literature screening
Two reviewers (LL and DCZ) independently conducted the literature search and screening independently. The selection processes were performed under the PICOS (Population, Intervention, Comparison, Outcome, Study design) framework, which involves five domains: research population, intervention, control measures, research outcomes, and study design. The reviewers screened the literature by browsing the titles and abstracts. Literature that satisfied the inclusion criteria was then determined by full-text intensive reading. Discrepancies were resolved through discussions, and when necessary, a third reviewer (Chen) was consulted until a consensus was reached.
Data extraction
Two researchers (LL and DCZ) independently extracted the following information from the eligible studies: first author, publication year, study location, sample size, type of surgery, age and follow-up time of the subjects, the measurement method of outcome indicators, generated randomization method, allocation concealment, blinding method, and use of intention-to-treat analysis. The Cochrane Quality Assessment Tool was applied to assess the risk of bias in the included RCTs, and the quality assessment involved random sequence generation, allocation concealment, blinding, loss of follow-up rates, selective reporting, and other biases (20). Due to a remarkable difference between the two interventions, it is impossible to implement the blinding method, and the evaluation of blinding is insignificant.
Outcome indicators
The primary outcome indicator was the rate of UTIs, and the secondary outcome indicators included the recovery of bladder functions, catheter duration, and endpoint residual urine volume.
Statistical analysis
Review Manager 5.4 (The Nordic Cochrane Centre, Copenhagen, 2020) was employed for literature quality assessment, and Stata 16.0 was used for data analysis. Cochran’s Q test and I2 index were applied to determine the heterogeneity among the studies. Subgroup analysis was performed according to the type of gynecological surgery, disease background, and follow-up time to explore the source of heterogeneity. When I2≤50%, the heterogeneity of the studies was not statistically significant, and a fixed-effects model was used for analysis; otherwise, a random-effects model was applied to pool the data. In terms of the continuous variables, due to the absence of measurement methods or dimensions, the weighted mean difference (WMD) was applied as an effect size to reflect the real experimental effect. Binary variables were reported as relative ratio (RR). The 95% confidence interval (CI) was provided for all evaluation indexes.
Sensitivity analysis was conducted on the outcome measures by excluding the studies one by one. This analysis can not only assess the robustness of the results but also can identify studies that had a greater impact on the study results, which may also be responsible for the heterogeneity. A funnel plot was created to assess the presence of publication bias in the included literature, and Egger’s or Begg’s tests were adopted for statistical testing (the number of studies was ≥8). For results with significant publication bias, the trim-and-fill method was employed to measure the impact of publication bias on the results. The types of surgery (radical resection of cervical cancer: 1; others: 0) or follow-up duration (14–21 days: 1; others: 0) were used as covariates included in the meta-regression analysis model (to ensure the reliability of the results, the number of studies should be ≥10) to determine the degrees and sources of heterogeneity of the studies so that it could provide a theoretical basis for subgroup analyses.
Results
Literature screening results
A total of 227 articles were retrieved from the seven described databases, of which 43 duplicates were excluded from the analysis. The titles and abstracts of the remaining 184 articles were subsequently reviewed, and 24 articles were available for full-text retrieval and further evaluation. Following the exclusion of five unpublished clinical trials (NCTs), 19 studies were included in the meta-analysis (21-39). The literature screening process is shown in Figure 1.
Research characteristics
The basic characteristics of the included studies are presented in Table 1. All of the included studies were published between 2011 and 2021, including two multicenter RCTs. Among the 19 included studies, articles on patients undergoing radical cervical cancer surgery accounted for the majority (n=13), followed by vaginal delivery (n=2), vaginal prolapse (n=1), gynecological pelvic surgery (n=1), endometrial cancer (n=1), and gynecological malignant tumors (including vulvar cancer, cervical cancer, ovarian cancer, and endometrial cancer) (n=1). The study sample sizes ranged from 56 to 180 participants.
Table 1
| Author | Year | Country | Source of patients | Intervention | Sample size | Age (years), mean ± standard deviation | Surgical procedure | Follow-up duration | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Cases | Controls | ||||||||
| Chen Q | 2020 | China | Single-center | CIC | TIC | 50 | 50 | 48.14±8.35 | 47.85±8.42 | Endometrial cancer | 1 week | ||
| Chen Y | 2021 | China | Single-center | CIC | TIC | 30 | 30 | 65.34±3.08 | 65.08±3.07 | Cervical cancer | 21 days | ||
| Feng P | 2017 | China | Single-center | CIC | TIC | 36 | 40 | 28.11±3.69 | 28.20±3.35 | Vaginal delivery | NM | ||
| Gao J | 2018 | China | Single-center | CIC | TIC | 66 | 66 | NS | NS | Cervical cancer | 3 months | ||
| Hakvoort RA | 2011 | Netherlands | Multi-center | CIC | TIC | 45 | 42 | 60±12 | 61±10 | Vaginal prolapse | NM | ||
| Liao D | 2020 | China | Single-center | CIC | TIC | 43 | 43 | 35.24±2.37 | 34.82±2.15 | Cervical cancer | 3 months | ||
| Lin Q | 2017 | China | Single-center | CIC | TIC | 30 | 26 | 48.7±9.2 | 49.3±10.3 | Gynecological malignant tumor | 14 days | ||
| Lin X | 2016 | China | Single-center | CIC | TIC | 60 | 60 | 42.2±10.9 | 40.6±12.4 | Cervical cancer | 21 days | ||
| Mei Z | 2020 | China | Single-center | CIC | TIC | 60 | 60 | 59.7±1.2 | 59. 2±1.4 | Cervical cancer | 21 days | ||
| Mulder FEM | 2018 | Netherlands | Multi-center | CIC | TIC | 40 | 45 | 30.7 | 30.3 | Vaginal delivery | 3 months | ||
| Peng Z | 2014 | China | Single-center | CIC | TIC | 43 | 40 | NS | NS | Cervical cancer | 14 days | ||
| Qian J | 2017 | China | Single-center | CIC | TIC | 38 | 38 | 48±9 | 47±10 | Pelvic surgery | 30 days | ||
| Ren M | 2019 | China | Single-center | CIC | TIC | 36 | 36 | 51.22±5.43 | 49.94±6.36 | Cervical cancer | 30 days | ||
| Wang X | 2021 | China | Single-center | CIC | TIC | 52 | 52 | 50.08±6.15 | 50.17±6.22 | Cervical cancer | 30 days | ||
| Xu X | 2018 | China | Single-center | CIC | TIC | 90 | 90 | 43.6±4.8 | 42.9±4.7 | Cervical cancer | 21 days | ||
| Yuan Y | 2019 | China | Single-center | CIC | TIC | 75 | 75 | 43.31±4.55 | 43.25±4.53 | Cervical cancer | 21 days | ||
| Zhan H | 2016 | China | Single-center | CIC | TIC | 43 | 36 | 44.45±13.21 | 45.61±11.28 | Cervical cancer | 14 days | ||
| Zhou J | 2021 | China | Single-center | CIC | TIC | 30 | 30 | NS | NS | Cervical cancer | 12 weeks | ||
| Zhu X | 2021 | China | Single-center | CIC | TIC | 49 | 51 | 49.67±10.07 | 48.06±9.55 | Cervical cancer | NM | ||
CIC, clean intermittent catheterization; TIC, transurethral indwelling catheterization; NS, no statistical difference; NM, not mentioned.
Quality evaluation
Our assessment of the risk of bias is shown in Figure 2. Fourteen studies had a lower risk of bias, whereas the remaining five articles had a higher risk of bias. In the studies with low risk of bias, most were due to the loss of follow-up (in two studies, the loss of follow-up rates were 10.8% and 18.8%, respectively), and the measurement bias of the outcome indicators was not described (12 studies). As for the five studies with a high risk of bias, their grouping was not all randomly classified; instead, convenience sampling, digital parity, and group sequential methods were applied. Reporting bias and other biases were not reported by all studies, and the blinding method was not applicable due to the significant difference between the intervention methods of the experimental and control groups.
Meta-analysis
This study conducted a meta-analysis on the 19 included studies, involving two interventions and four outcomes. Fifteen of the studies reported on the primary outcome of interest - the rate of UTIs, 10 documented endpoint residual urine volume, eight described recovery of bladder functions, and six studies stated the patients’ catheter duration.
UTI rate
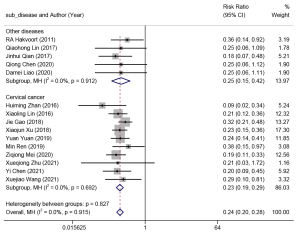
The fifteen studies that reported on the incidence of UTIs adopted the fixed-effect model (I2=0%, P=0.827), and the meta-analysis results indicated that clean intermittent catheterization substantially reduced the risk of UTIs (RR =0.24, 95% CI: 0.20 to 0.28) as compared with transurethral indwelling catheterization. Subgroup analyses were subsequently performed in terms of the context of conditions, and there was no statistical significance in the heterogeneity test either among the subgroups or within the subgroups (I2=0%, P>0.05) (Figure 3).
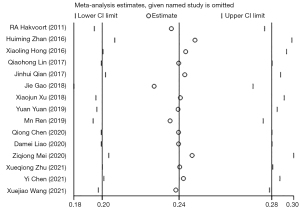
Sensitivity analysis illustrated the robustness of the current findings, which did not produce any alternation following the exclusion of each individual trial (Figure 4). In light of the funnel plots and Egger’s and Begg’s tests, publication bias yielded no substantial effect on the results.
Residual urine volume
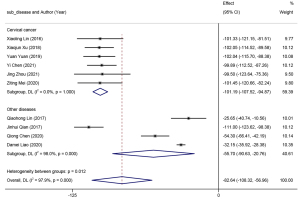
Ten studies reported on endpoint residual urine volume. A random-effects model (I2=97.9%, P=0.00) was adopted for meta-analysis. The results revealed that the residual urine volume was markedly lower in patients with urinary retention who received intermittent catheterization than those receiving indwelling catheterization (WMD =−82.64, 95% CI: −108.32 to −56.96). Subgroup analyses were then performed based on the type of surgery (Figure 5), and heterogeneity was determined among the subgroups (P=0.012). The subgroup study of radical cervical cancer resection yielded favorable homogeneity (I2=0.0%, P=1.00), whereas high heterogeneity was determined in the subgroup study of other types of gynecological surgery (I2=98.0%, P=0.00). The results of the subgroup meta-analysis implied that clean intermittent catheterization could achieve a lower residual urine volume in patients undergoing cervical cancer surgery compared to other conventional gynecological surgeries.
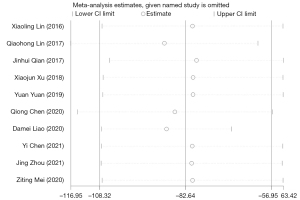
Additionally, after regression analysis, the association between the type of surgery and residual urine volume was statistically significant (P=0.020), which further validated the effect of different types of surgery on residual urine volume. Sensitivity analysis revealed that the results were robust and did not change after the exclusion of each individual trial (Figure 6). In light of the funnel plots and Egger’s and Begg’s tests, publication bias yielded a remarkable influence on the endpoint residual urine volume (P>0.05).
Recovery of bladder functions
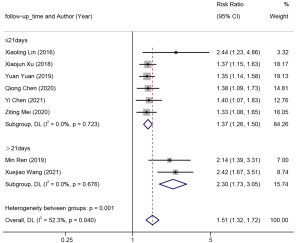
Eight studies reported the recovery of bladder functions, and a random-effects model (I2=52.3%, P=0.040) was adopted for meta-analysis. The results are shown in Figure 7. Clean intermittent catheterization had a higher recovery rate of bladder functions versus indwelling catheterization (RR =1.51, 95% CI: 1.32–1.72).
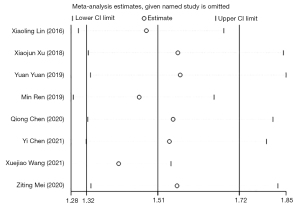
Patients with urinary retention who accepted different surgeries had different durations of bladder drainage; the optimal duration of catheterization after anterior colporrhaphy did not exceed two days, 2 to 3 days for ovarian cancer surgery, 1 to 3 days for vaginal delivery, 3 to 5 days for uterine prolapse surgery, 7 to 10 days for mild bladder injury, and 14 to 21 days for closed urethral injury and radical cervical cancer resection (40,41). The follow-up time of RCTs was scheduled mostly based on the duration of treatment. The actual efficacy of different measures cannot be identified if the follow-up time either exceeds or is less than the conventional treatment time. Thus, a rationally designed experimental cycle exerts an essential impact on improving the compliance of the subjects, reducing the loss of follow-up, and implementing the perfect allocation of medical resources, thereby saving more time and achieving the most benefits. Subgroup analysis of the follow-up time indicated favorable homogeneity within both subgroups (I2=0.0%, P>0.5), and there was certain heterogeneity between the subgroups (P<0.05). Thus, the follow-up time may be a major source of heterogeneity.
Sensitivity analysis indicated a robust result, as shown in Figure 8. The funnel plots and Egger’s test indicated the presence of significant publication bias. Due to the limited number of included studies, regression analysis was not conducted.
Duration of catheter maintenance
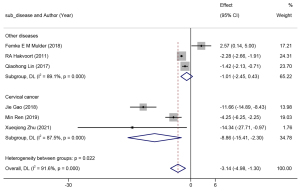
Six studies reported on the duration of catheter maintenance (days), and a random-effects model was adopted to combine the effect size (I2=91.6%, P=0.00). The results are shown in Figure 9. The catheter retention time for patients who received clean intermittent catheterization was substantially reduced compared to those with indwelling catheterization (WMD =−3.14, 95% CI: −4.98 to −1.30). Subgroup analysis of the type of surgery indicated significant heterogeneity between the subgroups and within each subgroup (I2>85%, P<0.05). The results of the subgroup meta-analysis revealed that a shorter duration of catheter maintenance was needed for patients who accepted cervical cancer surgery with clean intermittent catheterization (WMD =−8.86, 95% CI: −15.41 to −2.30), as compared to other routine gynecological surgeries.
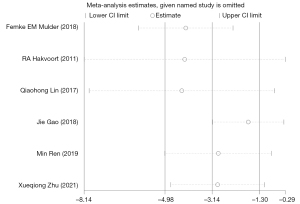
However, sensitivity analysis revealed that the result was not robust, as the results were altered when each individual trial was excluded (Figure 10). Funnel plots and Egger’s test indicated that the presence of publication bias had no significant effect on the results of catheter duration. Due to the limited number of included studies, regression analysis was not conducted.
Discussion
The present study demonstrated that clean intermittent catheterization performed better in reducing the risk of UTIs, improving the recovery of bladder functions, minimizing residual urine volume, and shortening catheter duration than conventional transurethral indwelling catheterization, which is consistent with the results of several RCTs (21-32). Our results are also in line with previous meta-analyses. For instance, in a 2018 meta-analysis and systematic review on the effect of different catheterization methods on UTIs after gynecological surgeries, the researchers reported that patients receiving clean intermittent catheterization have a lower incidence of UTIs than those receiving transurethral indwelling catheterization, which is consistent with our findings (18). However, this meta included only 2 RCT studies comparing intermittent catheterization and indwelling catheterization, and the rest focused on suprapubic catheterization. Due to the limited number of included studies, they only reported the pooled results of UTI, with a wider 95% CI, which may affect the reliability of their results. A 2017 network meta included people with urinary retention after orthopedic, urogynecological, gynecological, and general surgery, and compared the risk of UTI among intermittent catheterization, indwelling catheterization, and suprapubic catheterization. Four of its included studies compared the risk of UTI between intermittent catheterization and indwelling catheterization (1 cesarean section and 3 joint replacements). Although their study population and interventions were different from ours, their results also demonstrated the excellent performance of intermittent catheterization in reducing the risk of UTI. In addition, they found that intermittent catheterization and suprapubic catheterization had a lower risk of UTI than indwelling catheterization if the catheterization lasted more than 5 days (15). Another meta-analysis on the cost-effectiveness of different catheterization regimens for patients with urinary retention after pelvic surgery showed that cleaning intermittent catheterization is cheaper for short-term catheterization, followed by indwelling catheterization (requiring self-extraction of urinary catheter at home) (42).
UTIs
Previous studies have indicated that prolonged catheterization not only causes UTIs, but also increases the risks of a multitude of complications, namely bacteremia, urinary incontinence, UTIs, chronic kidney inflammation, and kidney stones (43,44). Among the included studies, 15 (79%) reported UTIs, which applied similar UTI definitions, and the basic characteristics of the subjects were balanced and comparable. Unfortunately, the application of antibiotics was not fully reported in all of such studies. Some early studies have reported that antibiotics have little or no effect in preventing UTIs for catheterization maintenance less than 3 days or more than 14 days (45). The experimental duration for a majority of studies included in our paper lasted 14–21 days, so there was no restriction on the application of antibiotics. Our meta-analysis indicated that clean intermittent catheterization could markedly reduce the risk of UTIs in patients with urinary retention after gynecological surgeries, as compared to the transurethral indwelling catheterization technique for bladder drainage (RR =0.24, 95% CI: 0.20 to 0.28). Furthermore, the regression analysis results revealed that the risk of UTIs was correlated with the choice of catheterization technique instead of the type of surgery (P>0.05).
A systematic review of 17 RCTs demonstrated that among three studies comparing transurethral indwelling catheterization and intermittent catheterization, there were fewer cases of bacteriuria in the clean intermittent catheterization group (RR =2.90, 95% CI: 1.44 to 5.84) (46). Another meta-analysis revealed that clean intermittent catheterization is associated with decreased symptomatic UTIs compared to transurethral indwelling catheterization (18). According to a systematic review on patients with urinary retention in benign prostatic hyperplasia, clean intermittent catheterization could reduce the risk of UTIs and accelerate spontaneous urination recovery, and may also be more cost-effective than transurethral indwelling catheterization (47). The previously described research results are consistent, providing theoretical support for further exploration of the application of clean intermittent catheterization in patients with urinary retention in various cases.
Residual urine volume
The gold standard for the diagnosis of urinary retention is a urination test after surgery, which is determined by the measurement of residual urine volume in the bladder. Normal urination is typically considered when the residual urine volume ranges from 100 to 200 mL. Some also diagnose urinary retention based on the criteria of bladder residual urine volume greater than 1/2 or 1/3 of the total bladder volume. The most stringent limit condition is that the residual urine volume of the bladder is less than 100 mL (2). In these included studies, residual urine volume is an important reference index to determine the implementation or termination of bladder drainage. In the 10 studies included in the meta-analysis, catheterization was terminated when residual urine volume was less than 100 mL. The results revealed that the residual urine volume in patients with urinary retention receiving clean intermittent catheterization was markedly lower than those with transurethral indwelling catheterization (WMD =−82.64, 95% CI: −108.32 to −56.96). Another meta-analysis examining the effect of clean intermittent catheterization in patients undergoing radical hysterectomy also yielded differential results for residual urine volume (WMD =−81.48, 95% CI: −107.68 to −55.28, P<0.001) (48). Additionally, both the subgroup and regression analyses indicated a positive association between the type of surgery and the residual urine volume (P=0.020). These findings might suggest that the application of clean intermittent catheterization in patients with cervical cancer surgeries was measured in a lower residual urine volume with a more favorable effect than other routine gynecological surgeries, which provides further evidence of the catheterization options for such patients after surgery.
Recovery of bladder functions
Among the included 19 studies, eight reported on the recovery of bladder functions, albeit under inconsistent criteria. Six studies used the final residual urine volume after the voluntary voiding test to evaluate the recovery of bladder function. A residual urine volume less than 100 mL indicates a good recovery of bladder function in patients; otherwise, the bladder function is not recovered. The urethral catheterization should be continued (27,29-31,33-34). The other two studies used the complete results of urodynamic examination to assess the recovery of bladder function (35,36). The urodynamic evaluation method encompasses multiple indicators like urethral manometry, pressure-flow rate, uroflow rate, bladder compliance, and bladder pressure volume, in addition to the residual urine volume. Hence, the inconsistency in the evaluation criteria for bladder function recovery may be an important source of the moderate heterogeneity in the results of our meta-analysis (I2=52.3%, P=0.040). We also elucidated whether the follow-up time affected the outcome of bladder function recovery. Subgroup analysis was subsequently conducted based on the follow-up time, the results indicated satisfactory homogeneity within each subgroup, and the follow-up time might be the main source of heterogeneity (P<0.05). Through further in-depth analysis of the included literature, this study found that in the subgroup with a follow-up time of more than 21 days, the follow-up time was up to 30 days, and the observational indicators included multiple indicators of urodynamics, such as bladder pressure volume measurement, bladder compliance, urethral pressure measurement, and urinary flow rate measurement. It is therefore inferred that longer follow-up time and more integrated indicators might be the main reasons for the presence of heterogeneity.
The pooled results of the random-effects model indicated that clean intermittent catheterization could improve the recovery of bladder functions compared to transurethral indwelling catheterization (RR =1.51, 95% CI: 1.32–1.72), which was consistent with a previous study (49). This might explain why long-term transurethral indwelling catheterization can weaken bladder tension and decrease detrusor contractility, resulting in patients not being able to urinate freely following the removal of the catheter. However, clean intermittent catheterization could promote the recovery of bladder muscle tension through proper filling and emptying of the bladder (48,50). Moreover, this conclusion was statistically significant in different subgroups. Due to the statistically significant differences between the subgroups, we speculated that better bladder recovery might be observed during a longer follow-up time. However, in terms of cost-effectiveness, an experimental duration ≤21 days is the best applicable time, which is helpful for designing a reasonable test cycle.
Duration of catheter maintenance
The duration of catheter maintenance is the main cause of hospital-acquired infection, and the daily risk of bacteriuria infection is between 3% and 7% (44). The duration of the catheter in the body not only increases the probability of UTIs and kidney damage but also produces a negative impact on the patient’s physiology and psychology (51,52). The clinical practice guidelines issued by the Infectious Diseases Society of America in 2009 proposed that the most effective way to reduce the incidence of catheter-related UTIs is to minimize catheterization as much as possible and remove the catheter immediately if not required (44). A Cochrane systematic review updated in 2021 also pointed out that shorter catheter duration may reduce the risk of catheter-related UTIs and dysuria (53).
The results of the included studies indicated that the duration of catheterization in patients who received clean intermittent catheterization was markedly lower than those with transurethral indwelling catheterization (WMD =−3.14, 95% CI: −4.98 to −1.30). Further subgroup analysis of the type of surgery indicated a statistical significance in the previously stated conclusions among different subgroups. Additionally, patients undergoing radical cervical cancer resection had shorter catheter durations with clean intermittent catheterization (WMD =−8.86, 95% CI: −15.41 to −2.30 vs. WMD =−1.01, 95% CI: −2.45 to −0.43). Previous studies have shown that the advantages of clean intermittent catheterization may link to differences in bladder training. Through intermittent bladder drainage and filling, the bladder can be stimulated early, allowing it to achieve early recovery of bladder functions. Conversely, the placement of an indwelling catheter postpones bladder stimulation, preventing an early start of bladder training, and thereby delaying the recovery of urodynamics to a normal level.
Despite the exciting results, high heterogeneity was also determined among and within the subgroups. Through sensitivity analysis and in-depth exploration, it was found that two studies might be the main source of heterogeneity. One study was conducted by Mulder et al. (22), which described the catheter days with clean intermittent catheterization using a median combined with a range. Of the 37 subjects in the control group in the study, ≤2 patients had a catheter duration of up to 32 days, whereas the remaining 35 patients had a catheter duration of ≤2 days. This study, which was described using a huge range, was the only one included in our meta-analysis that showed opposite results (WMD =2.57, 95% CI: 0.14 to 5.00); thus, it was reasonably assumed that this study was a major source of the heterogeneity. Another study that might have caused the heterogeneity was performed by Zhu et al. (37), which might be related to the randomization scheme of the research subjects. This study was grouped based on the parity of the hospitalization number, which might lead to the presence of a certain selection bias.
Limitations
This study has several limitations that should be noted. Firstly, our search was limited to four foreign databases and three domestic databases. Although the Web of Science, PubMed, Cochrane Library, and Embase databases are highly inclusive, there may still be missed literature. Secondly, most of the included studies were conducted in the Netherlands and China. More multicenter, large-scale RCTs are needed to demonstrate the effectiveness of the various drainage techniques. Thirdly, the current meta-analysis failed to offer more accurate and rigorous results using statistical analysis, as most of the literature did not report on the patients’ satisfaction and comfort, and the measurement methods adopted by the studies were not uniform. From the perspective of descriptive results, the comfort and satisfaction of patients who received clean intermittent catheterization were higher than those with transurethral indwelling catheterization.
Despite the aforementioned limitations, by synthesizing the evidence of 19 RCTs conducted in patients with urinary retention after gynecological surgery, we provided more inclusive evidence regarding the number of articles than previously published reviews (15,18). Meanwhile, our study also explored more outcome indicators for catheter duration and bladder function recovery and evaluated the effectiveness and safety of different bladder drainage techniques from multiple perspectives.
Conclusions
Clean intermittent catheterization can lower the incidence of UTIs, reduce residual urine volume, shorten the duration of catheter days, and improve bladder function recovery in patients with urinary retention after gynecological surgery. In particular, it might be more effective in patients undergoing radical cervical cancer resection. Further, clean intermittent catheterization might also yield a higher cost-benefit. However, due to the presence of local heterogeneity and the risk of biases, more studies with larger sample sizes are still needed to verify the findings of the current research.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist Available at https://tau.amegroups.com/article/view/10.21037/tau-23-220/rc
Peer Review File: Available at https://tau.amegroups.com/article/view/10.21037/tau-23-220/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-23-220/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zaĭtsev AN, Maganova NB. Embryotoxic effects of some aromatizers for food products. Vopr Pitan 1975;64-8. [PubMed]
- Geller EJ. Prevention and management of postoperative urinary retention after urogynecologic surgery. Int J Womens Health 2014;6:829-38. [Crossref] [PubMed]
- Liang CC, Chang SD, Chang YL, et al. Postpartum urinary retention after cesarean delivery. Int J Gynaecol Obstet 2007;99:229-32. [Crossref] [PubMed]
- Saultz JW, Toffler WL, Shackles JY. Postpartum urinary retention. J Am Board Fam Pract 1991;4:341-4. [PubMed]
- Yip SK, Sahota D, Pang MW, et al. Postpartum urinary retention. Acta Obstet Gynecol Scand 2004;83:881-91. [Crossref] [PubMed]
- Plotti F, Angioli R, Zullo MA, et al. Update on urodynamic bladder dysfunctions after radical hysterectomy for cervical cancer. Crit Rev Oncol Hematol 2011;80:323-9. [Crossref] [PubMed]
- Zullo MA, Manci N, Angioli R, et al. Vesical dysfunctions after radical hysterectomy for cervical cancer: a critical review. Crit Rev Oncol Hematol 2003;48:287-93. [Crossref] [PubMed]
- Ito E, Saito T. Nerve-preserving techniques for radical hysterectomy. Eur J Surg Oncol 2004;30:1137-40. [Crossref] [PubMed]
- Kim HS, Kim K, Ryoo SB, et al. Conventional versus nerve-sparing radical surgery for cervical cancer: a meta-analysis. J Gynecol Oncol 2015;26:100-10. [Crossref] [PubMed]
- Kietpeerakool C, Aue-Aungkul A, Galaal K, et al. Nerve-sparing radical hysterectomy compared to standard radical hysterectomy for women with early stage cervical cancer (stage Ia2 to IIa). Cochrane Database Syst Rev 2019;2:CD012828. [Crossref] [PubMed]
- Westberg SM, Pereira C, Rosdahl R, et al. Management of hypertension in pregnancy: a descriptive report of two clinic practices. Hypertens Pregnancy 2020;39:43-7. [Crossref] [PubMed]
- Wright MO, Kharasch M, Beaumont JL, et al. Reporting catheter-associated urinary tract infections: denominator matters. Infect Control Hosp Epidemiol 2011;32:635-40. [Crossref] [PubMed]
- Di Benedetto P. Clean intermittent self-catheterization in neuro-urology. Eur J Phys Rehabil Med 2011;47:651-9. [PubMed]
- Bakke A, Digranes A, Høisaeter PA. Physical predictors of infection in patients treated with clean intermittent catheterization: a prospective 7-year study. Br J Urol 1997;79:85-90. [Crossref] [PubMed]
- Han CS, Kim S, Radadia KD, et al. Comparison of Urinary Tract Infection Rates Associated with Transurethral Catheterization, Suprapubic Tube and Clean Intermittent Catheterization in the Postoperative Setting: A Network Meta-Analysis. J Urol 2017;198:1353-8. [Crossref] [PubMed]
- Groen J, Pannek J, Castro Diaz D, et al. Summary of European Association of Urology (EAU) Guidelines on Neuro-Urology. Eur Urol 2016;69:324-33. [Crossref] [PubMed]
- Gamé X, Phé V, Castel-Lacanal E, et al. Intermittent catheterization: Clinical practice guidelines from Association Française d'Urologie (AFU), Groupe de Neuro-urologie de Langue Française (GENULF), Société Française de Médecine Physique et de Réadaptation (SOFMER) and Société Interdisciplinaire Francophone d'UroDynamique et de Pelvi-Périnéologie (SIFUD-PP). Prog Urol 2020;30:232-51. [Crossref] [PubMed]
- Li M, Yao L, Han C, et al. The incidence of urinary tract infection of different routes of catheterization following gynecologic surgery: a systematic review and meta-analysis of randomized controlled trials. Int Urogynecol J 2019;30:523-35. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Hakvoort RA, Thijs SD, Bouwmeester FW, et al. Comparing clean intermittent catheterisation and transurethral indwelling catheterisation for incomplete voiding after vaginal prolapse surgery: a multicentre randomised trial. BJOG 2011;118:1055-60. [Crossref] [PubMed]
- Mulder FEM, Hakvoort RA, de Bruin JP, et al. Comparison of clean intermittent and transurethral indwelling catheterization for the treatment of overt urinary retention after vaginal delivery: a multicentre randomized controlled clinical trial. Int Urogynecol J 2018;29:1281-7. [Crossref] [PubMed]
- Peng Z, Huang L, Yu C, et al. Effect of clean intermittent catheterization for the treatment of urinary retention following radical resection of cervical carcinoma. Journal of Bengbu Medical College 2014;39:677-9.
- Zhan H, Hu C. Application of Self-cleaning Intermittent Urethral Catheterization in Cervical Cancer Patients with Urinary Retention after Extensive Hysterectomy. Military Nursing 2016;33:39-42.
- Qian J, Sun Y, Zhu X, et al. Effect of intermittent catheterization on recovery of spontaneous urination function after gynecological pelvic operation. Chinese Journal of Critical Care Medicine 2017;10:194-6. (Electronic Edition).
- Gao J, Zhang H, Liu L, et al. Application of home-based clean intermittent catheterization for rapid recovery of urinary bladder after radical surgery for cervical cancer. Journal of Nursing Science 2018;33:81-3.
- Lin X, Deng H, Liu D, et al. Application of clean intermittent self-catheterization to post-operative cervical cancer victims with urinary retention. Journal of Nursing Science 2016;31:58-9.
- Lin Q, Shi S, Zhuo L, et al. Effect of intermittent self-catheterization on postoperative urinary retention after surgery for gynecological malignant tumors. International Medicine and Health Guidance News 2017;23:3799-803.
- Xu X. Cervical cancer postoperative urinary retention take intermittent self-cleaning Effect analysis of urethral catheterization treatment. Chinese General Practice Nursing 2018;16:2240-2.
- Yuan Y, Wei W, Xiong Y. Analysis of the effect of intermittent self-cleaning catheterization for postoperative urinary retention in cervical cancer. Contemporary Medicine 2019;25:27-8.
- Chen Q, Li Y. Effects of clean intermittent self-catheterization management on incidence of postoperative complications in patients with endometrial cancer after radical resection. Chinese Journal of Clinical Oncology and Rehabilitation 2020;27:1129-31.
- Liao D, Huang F. Intermittent catheterization on urination in patients with neurogenic bladder after extensive total hysterectomy Effects of urodynamics. Modern Medicine and Health Research Electronic Journal 2021;4:19-22.
- Chen Y, Liu D. The extensiveness of self-cleaning intermittent catheterization for cervical cancer Effect analysis of patients with urinary retention after total hysterectomy. Women's Health Research 2021;(5):119-20.
- Mei Z. Application of intermittent self-cleaning catheterization in patients with urinary retention after cervical cancer surgery. New Mom and New Born 2020;(27):274.
- Ren M, Tu S, Guo S, et al. Application of cleaning intermittent catheterization in the fast rehabilitation of bladder function after radical hysterectomy for cervical cancer. Chinese Clinical Nursing 2019;11:288-91.
- Wang X, Yuan H. Observation on the effect of clean intermittent catheterization on bladder function rehabilitation after radical resection of cervical cancer. Chongqing Medicine 2021;50:892-5.
- Zhu X, Zhu H, Lv M, et al. Application of early intermittent catheterization in bladder management after radical resection of cervical cancer. Journal of Nursing and Rehabilitation 2021;20:57-9.
- Feng P, Chen C, Xie H, et al. To compare the effect of intermittent catheterization and indwelling catheterization in parturient women. Medical Innovation of China 2017;14:85-8.
- Zhou J, Zhang R, Tang X, et al. Effect of self-cleaning intermittent catheterization on bladder dysfunction in patients after radical hysterectomy for cervical cancer. Journal of Modern Medicine & Health 2021;37:4273-7.
- Huang CC, Ou CS, Yeh GP, et al. Optimal duration of urinary catheterization after anterior colporrhaphy. Int Urogynecol J 2011;22:485-91. [Crossref] [PubMed]
- Bouhours AC, Bigot P, Orsat M, et al. Postpartum urinary retention. Prog Urol 2011;21:11-7. [Crossref] [PubMed]
- Wang R, Tunitsky-Bitton E. Short-term catheter management options for urinary retention following pelvic surgery: a cost analysis. Am J Obstet Gynecol 2022;226:102.e1-9. [Crossref] [PubMed]
- Chenoweth CE, Saint S. Urinary tract infections. Infect Dis Clin North Am 2011;25:103-15. [Crossref] [PubMed]
- Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010;50:625-63. [Crossref] [PubMed]
- Catheter-acquired urinary tract infection. Lancet 1991;338:857-8. [Crossref] [PubMed]
- Kidd EA, Stewart F, Kassis NC, et al. Urethral (indwelling or intermittent) or suprapubic routes for short-term catheterisation in hospitalised adults. Cochrane Database Syst Rev 2015;2015:CD004203. [Crossref] [PubMed]
- Baboudjian M, Peyronnet B, Boissier R, et al. Best nonsurgical managements of acute urinary retention: what's new? Curr Opin Urol 2022;32:124-30. [Crossref] [PubMed]
- Ma S, Xin L, Guo X, et al. Meta-analysis of the Effect of Intermittent Catheterization in Patients after Radical Resection of Cervical Cancer. Sichuan Medical Journal 2019;40:1241-9.
- Ghalayini IF, Al-Ghazo MA, Pickard RS. A prospective randomized trial comparing transurethral prostatic resection and clean intermittent self-catheterization in men with chronic urinary retention. BJU Int 2005;96:93-7. [Crossref] [PubMed]
- Li C, Wang J, Liu X. Effects of different ways of catheterization on urinary tract infection and bladder function in patients with spinal cord injury: a meta-analysis. Chinese General Practice Nursing 2021;19:1468-73.
- Saint S, Lipsky BA. Preventing catheter-related bacteriuria: should we? Can we? How? Arch Intern Med 1999;159:800-8. [Crossref] [PubMed]
- Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis 2001;7:342-7. [Crossref] [PubMed]
- Ellahi A, Stewart F, Kidd EA, et al. Strategies for the removal of short-term indwelling urethral catheters in adults. Cochrane Database Syst Rev 2021;6:CD004011. [PubMed]
(English Language Editor: A. Kassem)




