Interstitial cystitis intravesical therapy
Overview
Interstitial cystitis/painful bladder syndrome (IC/PBS) is a chronic, progressive disorder first described in the turn of the century by Boston gynaecologist, Guy Hunners who defined the characteristics of the disease (1). He also described the “Hunner Ulcer”, which is the typical cystoscopic finding of classic IC. There is now increasing evidence that there are two subtypes, classic inflammatory IC and non-ulcerative IC.
Patients characteristically present with urinary urgency, frequency, nocturia and pain related to bladder filling. The pain is located in the lower urinary tract—involving the suprapubic and pelvic area, perineum, vagina and urethra. In the early stages, IC presents with cycles of flares and remission. Flares are triggered by stressors, be it emotional, physical or hormonal fluctuations to name a few. These symptoms gradually deteriorate over time as the disease progresses.
Diagnosis is usually one of exclusion, and the NIDDK inclusion and exclusion criteria can be helpful in making a clinical diagnosis of IC (outlined in Table 1) (2) though these were developed initially to permit a unified research framework.

Full table
The aetiology of IC is still uncertain. In 1996, Parsons postulated that the pathophysiology of the disease involves an epithelial dysfunction of the lower urinary tract (3). He further described in 2003 that this dysfunction was primarily located in the glycosaminoglycans (GAG) layers of the bladder urothelium. The GAG layer is made up of hyaluronic acid (HA), chondroitin sulphate, heparin sulphate and keratin sulphate (4). In healthy patients, the GAG layer acts as a barrier to protect the bladder submucosa from urinary solutes. In IC, the GAG layer is damaged and made permeable, allowing an influx of urinary solutes, particularly potassium into the submucosa. This influx of potassium causes a cascade of tissue inflammation, degranulation of mast cells, and depolarisation of the sensory nerves, resulting in injury to tissue and bladder pain.
It is postulated that potassium is the urinary metabolite primarily responsible for generating bladder symptoms when the GAG layer is dysfunctional. The level of potassium required to depolarise nerves and muscles sits around 8–10 mEq/L, which is significantly lower than the K+ level in urine ~30–120 mEq/L. Thus, it is understandable that in IC, diffusion initiates a big cascade of depolarisation, generating symptoms of urgency, frequency, pain and incontinence (5).
Treatment strategies focus on:
- Restoring lower urinary tract epithelial function;
- Inhibiting neural activation;
- Controlling allergies;
- Relieving symptoms.
Current therapy involves behavioural modification, oral medical therapy, intravesical therapy and surgery. First line therapy involves oral medication and behavioural modification (e.g., pelvic floor exercises, controlled fluid intake and bladder training). Oral medical therapy encompasses analgesics (i.e., amitriptyline, gabapentin), antidepressants (tricyclic antidepressants), antihistamines, immunosuppressants and oral pentosan polysulfate. Second line therapy, involves the trial of intravesical therapy.
In this review, we will be focussing on current intravesical therapy and what lies on the horizon for IC. Intravesical instillation has the advantage of providing high concentrations of therapy locally in the bladder, whilst avoiding systemic side effects associated with oral medications. Unfortunately, very few large double-blinded randomised controlled studies exist in this field. This is largely due to difficulty double-blinding participants and the lack of an objective measure of response to treatment—success of a treatment is subjective to a patient’s personal assessment. This is in addition to the exacerbating and remitting nature of the disease.
Challenges to intravesical therapy include:
- Short duration of action;
- Lack of permeability of the bladder epithelium;
- Lack of bladder uptake of drugs.
Intravesical agents studied to date
Bacillus Calmette-Guerin (BCG)
IC is thought to be an autoimmune condition, with reports of autoantibodies being found in IC bladders and IC histopathology displaying similarities to other autoimmune condition. Patients with IC have been found to have 5 times the level of interleukin 6 productions compared to controls (6). This forms the basis behind BCG therapy. The exact mechanism of BCG therapy is not yet known. Theoretically it is believed to cause a down regulation of the interleukin-6 (IL-6) response which is upregulated in IC (7). This theory is challenged by the other studies reporting intravesical BCG increasing IL-6 in classic IC and worsening the inflammatory response (8).
Numerous studies have been performed, with the last randomised controlled study of 265 participants finding no difference between intravesical BCG therapy and placebo (9). Patients were randomised into 6 weekly instillations of placebo (50 mL normal saline) or BCG (1 mL BCG in 49 mL saline) and followed for 34 weeks. Whilst there were small improvements noted these differences were of little statistical significance. In addition, participants were commonly found to experience adverse systemic symptoms (e.g., arthritis) which subsided once treatment was ceased. In rare cases, BCG has been reported to cause life threatening septic reactions (10).
With significant risks and a low therapeutic benefit, BCG therapy has been phased out as an effective means of treating IC. As shown in Table 2, The American Urological Association (AUA) has classed this as an −A therapy, recommending against its use due to these downfalls (11). Of the intravesical agents this is perhaps the most comprehensively studied highlighting the early stage in the evolution of this disease and its therapies.
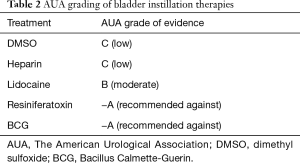
Full table
Dimethyl Sulfoxide (DMSO)
Similar to BCG, the mechanism of action of DMSO is not fully known. It is thought to be a scavenger of intracellular hydroxyl radicals which are postulated to be important triggers of the inflammatory response. DMSO has also been reported to stimulate nitric oxide release from afferent neurons, possibly reflecting the desensitisation of nociceptive pathways in the urinary tract (12).
These mechanisms work to reduce inflammation, relax the detrusor muscle, provide mast-cell inhibition and dissolve collagen. It may also cause temporary urothelial injury, potentially allowing better penetration for other intravesical agents and is being increasingly used in conjunction with other agents. Thus far, no combination of agents has been found superior to another. Currently, it is the intravesical treatment of choice in IC.
The treatment regime traditionally involves weekly bladder instillations of 50 mL of 50% DMSO solution for 6–8 weeks. The solution is retained in the bladder for 10–20 minutes at each instillation. After the initial course, treatment is suspended until a recurrence occurs.
The main side effect is a “garlic” breath odour for 2 days post instillation. Patient also report pain on instillation and catheter irritation (13). Initially, patients may notice a flare-up of symptoms but this settles throughout the course of treatment. Studies have found a 50% response rate with DMSO intravesical therapy and it is currently the mainstay of treatment.
Only two randomised controlled trials have been performed thus far to assess DMSO outcomes. Perez-Marrero et al. randomised 33 patients with IC to 50 mL of 50% DMSO therapy or saline placebo (14). Subsequently fortnightly instillations were given, for a total of four sessions and then participants were assessed. Primary outcomes measured were pain, urgency and bladder capacity. Ninety-three percent of DMSO vs. 35% of placebo patients noted objective improvements. Subjectively 53% of DMSO patients noted significant improvement compared to 13% of placebo patients. This 1988 study set the precedent for DMSO use for IC.
In 2000, Peeker et al. compared DMSO to BCG therapy (7) in a prospective, double-blind study. Twenty-one patients were randomised to 50 mL of 50% DMSO or 1 mL BCG (in 49 mL saline); solutions were given in six weekly sessions. Improvements were noted in the DMSO group in both urinary frequency and pain for classic IC, whereas nil improvement was noted in the BCG group. There was an improvement in VAS pain score for non-ulcerative IC after DMSO, but no change in other study outcomes. No improvement was noted in maximal functional capacity for both therapies.
The evidence at the moment is supportive of DMSO therapy for the management of IC but it is not conclusive. In practice, only a proportion of patients respond and mostly the responses are partial rather than complete or permanent. This is likely due to the multifactorial and progressive nature of the disease although DMSO is a non-specific therapy targeting a complex chronic inflammatory disease. In an attempt to target the different components of IC and its manifestations, multiagent intravesical therapy has been trialled (15), where 65.5% of IC patients responded to 50% DMSO, 100 mg hydrocortisone and 25,000 IU heparin in eight weekly instillations. Of note, high levels of treatment failure were noted in patients with advanced disease states—that is, those who had severe cystoscopic glomerulations, microscopic haematuria and urodynamic detrusor underactivity. Thus, other more effective avenues of treatment still need to be explored.
Vanilloids
The bladder pain experienced in IC is believed to be mediated by afferent C-fibre sensory neurons—with 50% of bladder afferents being unmyelinated sensory C fibres. These C-fibres may also have a mechanosensitive role in contributing to the urinary urgency and frequency seen in IC. In IC, these fibres are abnormally stimulated due to urothelium injury. The potential of vanilloids (i.e., resiniferatoxin, capsaicin) was first suggested by in-vitro data which demonstrated C-fibres entering a refractory state and becoming desensitised after vanilloid stimulation (16). This desensitisation in pain fibres was hoped to translate into clinical management of IC.
Research has been performed into vanilloid receptor agonists. The theory is that the use of these agents will desensitise C-fibres and therefore provide symptomatic pain relief. Currently trials in these two agents have been unfruitful.
Resiniferatoxin
Resiniferatoxin is a potent analogue of Capsaicin, which has been found in animal models to have improved efficacy to pain fibre receptors (17).
In 2005, Payne et al. randomly allocated 163 participants to receive one dose of intravesical 50 mL resiniferatoxin (at differing doses of 0.01, 0.05 and 0.10 mcg) or a placebo (17). Twelve weeks post intravesical instillation patients were evaluated using the Global Response Assessment (GRA). At the conclusion of the study, patients who underwent resiniferatoxin treatment demonstrated no improvement in symptoms. Additionally, significant post-instillation pain was noted with resiniferatoxin which corresponded to the dose given. This suggested that vanilloids may not be an appropriate treatment.
Payne et al.’s study is the largest resiniferatoxin study to date. A 2013 meta-analysis performed by Guo et al. (16), reviewed six other studies with smaller cohorts between 18–54 participants. These studies found no improvement in urinary frequency, nocturia, incontinence or bladder capacity but bladder pain was found to be reduced.
Botulinum toxin A
Botulinumtoxin is a potent neurotoxin produced from clostridium botulinum. It acts by cleaving the 25-kDa synaptosome-associated protein (SNAP-25 protein) in the presynaptic terminal (18). This prevents neurotransmission at the presynaptic membrane, which disables neural transmission from nerve fibres to bladder urothelium, preventing muscle contraction. Its muscle relaxant properties are believed to provide symptomatic relief in IC, but evidence suggests that it may carry analgesic properties as well. In 2009, the FDA introduced new drug names for botulinum toxin type A to reflect the differences in potencies. Whilst all inherently botulinum toxin A—onabotulinumtoxin A, abobotulinumtoxinA and rimabotulinumtoxin B all have different potencies and therefore, different indications for use.
The current evidence is non-conclusive. There is also no evidence to suggest that this therapy is sustainable. Efficacy is reduced with repeated injections raising concerns for long term safety and resistance with repeated administration.
In 2014, Manning et al. published a randomised, double blind study involving 54 women with severe IC (19). Participants were randomly allocated to either hydrodistension + intravesical instillation of Abobotulinum A (AboBTXA) or hydrodistension + intravesical instillation of normal saline. Outcomes were reviewed at 3 months via the O’Leary-Sant questionnaire. Results were confounded by 12 patients suffering from urinary tract infections, and these patients were not included in the final results. At the conclusion of the study, five AboBTXA participants showed >50% improvement compared to two placebo participants. The study concluded Abobotulinumtoxin A showed no overall improvement for patients suffering from severe refractory IC, but significant benefit was noted in a small number of patients.
Conversely, a larger 2015 retrospective study by Gao et al. demonstrated positive outcomes post Botulinumtoxin A instillation (18). A total of 124 women over a 10-year period [2003–2013] were retrospectively reviewed; 66 women were treated with 100 U of Botulinumtoxin A whilst 58 underwent bladder hydrodistension + sodium hyaluronate instillation. Again, participants were evaluated with the O’Leary-Sant or VAS score. At 1 week, 92.2% efficacy was noted after a single dose, though efficacy fell to 75% at 3 months. At 12 months, after a single dose, symptoms had returned to baseline, demonstrating no sustainability of this treatment but short term relief was achievable. The efficacy for Botulinumtoxin A was similar to the hyaluronate used as a control, demonstrating that it could be a potential safe and therapeutic alternative treatment for IC.
Both studies have their different merits, but to establish whether Botulinumtoxin A is of use in IC, a larger randomised controlled double blind trial is required.
Lidocaine
Lidocaine (xylocaine or lignocaine) is a common topical anaesthetic which currently has level B evidence for IC (AUA guidelines). It has shown promise as a short-acting analgesic in IC, with its effects rarely lasting past two weeks. Lidocaine is believed to anaesthetise the bladder afferent nerves which cause the lower urinary tract pain associated with IC. It is usually alkalinised to counter the acidic nature of the urine. Once instilled, the solution is buffered by surrounding tissue and penetrates the bladder lining by converting into a lipid-soluble base form.
One of the largest multi-centre, double blind, placebo-controlled studies was performed by Nickel et al. in 2008 (20). A total of 102 patients were randomised to receive 10 mL of 200 mg of alkalinised lidocaine (AL) or saline for 5 consecutive days. Outcomes were measured using the GRA scale, with patients being followed up to 29 days post-instillation. Thirty percent of experimental patients noted immediate improvement post-lidocaine course compared to 9.6% in the control group, but efficacy dropped off back to baseline by day 10. Lidocaine has been increasingly used in combination with other intravesical therapies due to its immediate efficacy, in particular heparin which is discussed later in this chapter .
GAG layer reconstruction
In recent years, GAG layer replenishment (21) has come to the forefront of IC management. First identified in 1975 by Parsons et al., the GAG layer is a mucus layer over the bladder urothelium that acts as a barrier against bacteria (22). It is made up predominantly of HA, chondroitin sulfate, heparin sulfate and keratin sulfate. Therefore, the theory behind GAG replenishment therapy is to reconstruct this layer by instilling components of the GAG layer into the bladder.
HA
HA is a glycoprotein that is an important component of the GAG layer. It alleviates the inflammatory process by both inhibiting leukocyte migration/aggregation and binding to lymphocytes and endothelial cells, blocking the ICAM-1 receptors (23). Additionally, it appears to inhibit mast cell degranulation, the activation of which is a crucial step in the natural history of IC (24).
Intravesical instillation of HA has shown some promise in small cohort trials, but no multi-centre, double blinded controlled study has shown any statistically significant benefit. In 2013, Lai et al. conducted a prospective randomised study comparing different HA treatment regime (25). Sixty patients were assigned to receive 4 weekly instillations, followed by 5 monthly instillations (HA-9 group) or 12 instillations fortnightly (HA-12 group). Both groups showed improvement in O’Leary Sant scores, VAS score and quality of life. No statistically significant difference between regimes was noted though the data below demonstrates a trend to improvement occurred in all parameters with increasing numbers of instillations.
Due to the multifactorial aetiology of IC, patients are being increasingly treated with combined intravesical therapy to potentiate the therapeutic benefit of treatment courses. Lv et al. compared the effectiveness of combined HA with AL to two control groups of monotherapy (26). Forty-five women were randomised, with the combined HA + AL group demonstrating significant improvement at week 2 which continued onto trial completion at 48 weeks. Monotherapy AL showed improvement at 2 weeks, but therapeutic benefit ceased at week 24, whilst monotherapy HA had a later onset at 4 weeks but remained beneficial until study completion.
Chondroitin sulfate
Chondroitin sulfate has been identified as a key component in the human bladder GAG layer. Theoretically, instilling chondroitin, would be expected to be more beneficial than HA when used to regenerate the GAG layer due to the larger role it plays.
A course involves 20 mL of 2.0% sodium chondroitin sulfate instilled into the bladder weekly for 6–8 weeks.
A 2009 open study by Nickel et al. treated 53 patients with sodium chondroitin weekly for 6 weeks, then monthly for 4 months—a total of 10 treatments (27). Outcomes were compared to baseline function and 60.4% of patients noted improvement in pain, urgency and frequency at the 24-week mark, demonstrating potential efficacy for this treatment.
Nickel et al. subsequently went on to review the efficacy of sodium chondroitin in a randomised, double blind cohort, by comparing outcomes to an inactive normal saline control group (28). Patients were treated with weekly instillations for 6 weeks, then followed up for a further 6 weeks. An improvement was noted in 22.6% of control patients vs. 39.4% in the chondroitin group.
A second randomised control study was then performed by Nickel et al. in 2012 with a larger cohort of 98 women (29). They were randomised to receive sodium chondroitin therapy or an inactive control for 8 weeks. Thirty-eight percent of the active group noted improvement compared to 31.3% in the control group. As these results were not statistically significant, there does not appear to be a benefit to this monotherapy.
Combined chondroitin + HA (iAluRil)
Although there is no support for Sodium Chondroitin efficacy, combined therapy of 1.6% hyaluronic and 2% chondroitin intravesical instillation therapy has shown promise. It is currently marketed as iAluRil.
A course of iAluRil consists of 40 mL of sodium hyaluronic 1.6% and chondroitin sulfate 2.0% in normal saline. This mixture is instilled intravesically weekly for 8 weeks, then as required (24).
A 2008 prospective, open and uncontrolled study by Cervigni et al., followed 23 women who received weekly instillations of iAluRil for 20 weeks, followed by monthly instillations for 3 months (23). Patients were then followed up for 5 months post completion of treatment to determine sustainability. Outcomes were measured using the O’Leary-Sant index, voiding diaries and Visual Analogue scales. Improvements were noted in frequency, urgency and pain.
These findings were corroborated by Porru et al. in a 2011 study with 22 patients who received 8 weekly instillations, followed by fortnightly for 6 months (24). Voiding volumes, urinary urgency and pain all improved, though in varying degrees, in all patients.
In 2012, Cervigni et al. published the long term outcomes of iAluRil over a period of 3 years (30). Twelve patients were treated over a 3-year period and evaluated with positive findings. Patients noted improvements in frequency, void volumes and quality of life.
These results indicate that sodium chondroitin is of benefit when used in combination with HA, but is not beneficial as a monotherapy. Of note, iAluRil studies have not noted any significant side effects with the use of this combined therapy.
Further studies are required with larger controlled studies to validate these results but there appears to be promise with GAG targeted therapy.
Heparinoid compounds
Heparin + AL
Heparin is a polysaccharide which is one of the components of the GAG layer. Similar to the above GAG therapies, heparin acts as an endogenous GAG when it is instilled intravesically. Theoretically, it restores some of the bladder urothelium’s natural function, which is damaged in IC.
A typical course of heparin involves instilling 10,000–40,000 IU in 10 mL of water, 3 times a week with a retention time of 1 hour. Patients are treated for 3 months, with further maintenance therapy offered if improvement is noted (11).
In 1994, Parsons et al. treated 48 patients with the above intravesical heparin regime (31). Results were positive with 56% reporting improvement, though no long term follow-up was recorded. No randomised controlled study has been performed on heparin monotherapy to verify these results.
Intravesical heparin therapy does not provide immediate symptom relief, therefore it is being increasingly paired with AL which provides short term relief in the interim. The lidocaine is alkalinised to avoid ionisation with the acidic urine. AL is postulated to readily diffuse through the bladder epithelium and anaesthetise the afferent sensory C-fibres. Heparin potentiates the efficacy of lidocaine by coating the bladder wall and blocking potassium efficacy.
Parsons et al. conducted a multicentre prospective, double blind, crossover study in 2012 with heparin + lidocaine with positive results (5). Eighteen patients underwent a double-blind controlled study, where they were randomised to receive either 50,000 units of heparin, 200 mg of lidocaine and 420 mg sodium bicarbonate in 15 mL of water or 420 mg of sodium bicarbonate in 15 mL of water. A 42% reduction in pain was noted in the immediate time period.
Currently the long term sustainability and benefit of heparin is unknown.
Pentosan polysulfate (PPS)
Traditionally, PPS is given as an oral agent at a dose of 300 mg/day for 8 months. It is the only FDA approved IC drug in America. Studies experimenting with intravesical PPS instillation have shown some promise, but cohorts have been small and a large randomised controlled study is required. The theoretical benefit postulates as PPS is similar in structure to the GAG layer, this agent may help restore the urothelial mucosal layer.
When instilled intravesically, 200–300 mg of oral PPS is dissolved in 30 mL of normal saline. The solution is administered weekly for a total of 6 weeks, with top up therapy given as required. Davis et al. performed a blinded trial involving 41 women who were randomly allocated to be given oral and intravesical PPS or oral PPS and intravesical placebo of normal saline (32). Six weeks of weekly intravesical therapy were given, and oral PPS was continued for 12 weeks in both groups. The O’Leary Sant index was used to measure outcomes. A 46% reduction in O’Leary Sant score was noted in the therapeutic group in contrast to the 24% reduction in the control group. At week 18, this improvement was noted to be sustained in the treatment group.
Similarly, Daha et al. published an open, uncontrolled study involving 29 women who underwent intravesical PPS therapy (33). Improvements in O’Leary Sant score were noted after 10 biweekly treatments of 300 mg intravesical PPS. This supports the theory that intravesical PPS can be of therapeutic value, though further higher level of evidence research is required.
New therapies on the horizon
The latest advance in intravesical therapy for interstitial cystitis involves the use of intravesical liposomes. In other aspects of medicine, liposomes have been found to have wound healing properties by providing a moisture film on the wound, without promoting an inflammatory reaction. Biophysical studies have found that liposomes can be adsorbed, fuse or transfer lipids to the cell membrane, as well as endocytose to the cell interior. Certain constituents of liposomes have been demonstrated to contribute to epithelial membrane impermeability, as well as modulate inflammation.
Liposomes
Liposomal therapy has become increasingly promising for potential mainstream application (34). Liposomes are essentially fat bubbles formed by phospholipid bilayers which contain water.
They theoretically create a film onto the bladder urothelium when instilled, forming a mechanical barrier. It has been found to decrease inflammation and irritation in IC bladders (35) and is postulated to restore the GAG layer with a 50% response rate (11).
The current regime for liposomal treatment is a weekly instillation of 80 mg of preliposomal sphingomyelin lyophilate with 40 mL of sterile water (36) for 4 weeks. The solution is retained in the bladder for 30 minutes or more before being evacuated by the patient after catheter removal.
Two promising studies have been performed with liposomes, the first being Chuang et al. in 2009 (37) where the response of intravesical liposome therapy was compared to oral PPS. Twenty-four patients were prospectively followed, half had 80 mg of liposomal therapy instilled for 4 weeks whilst the other treatment arm was given 300 mg of oral PPS daily. Outcomes were measured using the O’Leary Sant score, visual analog and GRAs at 4 and 8 weeks post treatment. Statistically significant improvements (50% of participants) in urinary urgency and pain were noted at 4 weeks, which remained effective at 8 weeks. Urinary frequency and nocturia scores were comparable to the PPS group.
Peters et al. in 2014 (36) followed 14 IC patients who underwent weekly instillations of liposomes for 4 weeks. Outcomes were measured via the VAS (visual analog scales), O’Leary Sant Index and GRAs. Cystoscopic improvements were noted in three participants, no change in 10 and worsening in 1 participant. No adverse side effects were noted and outcomes were positive with improvements in pain, urgency and overall symptoms scores. Improvements in urgency were sustained at 8 weeks. Although pain scores significantly improved at 4 weeks, they were found to be back to baseline at 8 weeks.
With no reportable side effects and a relatively high response rate, liposomal therapy requires further investigation to determine if the 50% response rate is repeatable in a larger randomised control study. With similar response rates to PPS, intravesical instillation of liposomes provides an avenue which bypasses potential systemic side effects or allergies whilst providing high local drug concentrations at the site of injury.
Conclusions
The aetiology of IC is not well understood. It is postulated that bladder urothelium dysfunction causes the chronic inflammation and bladder hypersensitivity seen in IC. Current therapies have attempted to target this GAG layer, but no study to date has been associated with a full remission. This suggests at best only partial repair to the damaged barrier. Because the agents are not directly anti-inflammatory they may not reverse the secondary submucosal inflammation and thus, have to-date failed to fully eradicate bladder pain.
The initial assault is believed to be caused by an infection. A breakthrough in this theory occurred when Zhang et al. from China isolated nanobacteria in bladder tissue samples of IC and noted symptom improvement and decreased levels of nanobacteria after tetracycline treatment (38). Lower concentrations of uromodulin and kininogens, and higher levels of intertrypsin inhibitor H4 were also revealed on proteomic analysis in patients with IC. With this breakthrough, the future of IC therapy may be heading towards targeting these proteins. However, it is important to note that systematic use of anti-microbials has been ineffective.
Other studies have found IC urine contain increased levels of antiproliferative factor which plays a role in inhibiting epithelial cell proliferation in the bladder (39). IC bladders also appear to contain epithelial cells which produce an inhibitor of heparin-binding epidermal growth factor-like protein. Both these mechanisms reduce urothelial proliferation in the IC bladder. This inhibition is possibly modulated by currently unidentified genes.
IC/BPS is a multifaceted condition with likely multiple aetiologies and pathological pathways. It is little wonder that many therapeutic efforts seem to be effective for some and not others. In the spectrum of BPS, IC is still a pathophysiological enigma and its treatment empirical, though it fits within the paradigm of chronic regional pain syndrome. It is considered a multifactorial cascade of events that culminate into an imbalance of the damage-repair process of the urothelium, leading to deficiency of the GAG layer and resulting symptoms. From the abnormal urothelial permeability, sensory nerve stimulation and mast cell activation, this complex process contributes to the chronicity of IC and the unsatisfactory response to treatment.
Despite widespread empirical use of intravesical agents, the evidence of efficacy is in an early phase of its evolution. Studies such as the BCG trials do show it is possible to conduct RCT studies in the patient cohort. Most studies are uncontrolled, with small numbers and open methodology. There is a paucity of large randomised controlled trials in this field, the gold standard in clinical research, thus not yet enabling us to fully relieve suffering in patients with IC.
Acknowledgements
We would like to acknowledge Mr. J. R Sathiyananthan, consultant urologist and general surgeon, for his technical expertise during the writing of this review.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hunner GL. A rare type of bladder ulcer in women; report of 8 cases. Boston Med Surg J 1915;172:660-4. [Crossref]
- Hanno P, Baranowski A, Fall M, et al. Painful bladder syndrome (including interstitial cystitis). In: Abrams P, Cardozo L, Khoury Saad, et al. editors. Incontinence Vol 2: Management. 3rd edition. Paris: International Continence Society;2005:1473-4.
- Parsons CL. Interstitial cystitis. Int J Urol 1996;3:415-20. [Crossref] [PubMed]
- Hurst RE, Roy JB, Min KW, et al. A deficit of chondroitin sulfate proteoglycans on the bladder uroepithelium in interstitial cystitis. Urology 1996;48:817-21. [Crossref] [PubMed]
- Parsons CL, Zupkas P, Proctor J, et al. Alkalinized lidocaine and heparin provide immediate relief of pain and urgency in patients with interstitial cystitis. J Sex Med 2012;9:207-12. [Crossref] [PubMed]
- Lotz M, Villiger P, Hugli T, et al. Interleukin-6 and interstitial cystitis. J Urol 1994;152:869-73. [Crossref] [PubMed]
- Peeker R, Haghsheno MA, Holmäng S, et al. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis: a prospective, randomized double-blind study. J Urol 2000;164:1912-5; discussion 1915-6.
- Jackson AM, Alexandroff AB, Kelly RW, et al. Changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-1) in bladder cancer patients after bacillus Calmette-Guérin (BCG) immunotherapy. Clin Exp Immunol 1995;99:369-75. [Crossref] [PubMed]
- Mayer R, Propert KJ, Peters KM, et al. A randomized controlled trial of intravesical bacillus calmette-guerin for treatment refractory interstitial cystitis. J Urol 2005;173:1186-91. [Crossref] [PubMed]
- Paterson DL, Patel A. Bacillus Calmette-Guerin (BCG) immunotherapy for bladder cancer: review of complications and their treatment. Aust N Z J Surg 1998;68:340-4. [Crossref] [PubMed]
- Colaco MA, Evans RJ. Current recommendations for bladder instillation therapy in the treatment of interstitial cystitis/bladder pain syndrome. Curr Urol Rep 2013;14:442-7. [Crossref] [PubMed]
- Birder LA, Kanai AJ, de Groat WC. DMSO: effect on bladder afferent neurons and nitric oxide release. J Urol 1997;158:1989-95. [Crossref] [PubMed]
- Forrest JB, Dell JR. Successful management of interstitial cystitis in clinical practice. Urology 2007;69:82-6. [Crossref] [PubMed]
- Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. J Urol 1988;140:36-9. [Crossref] [PubMed]
- Hung MJ, Chen YT, Shen PS, et al. Risk factors that affect the treatment of interstitial cystitis using intravesical therapy with a dimethyl sulfoxide cocktail. Int Urogynecol J 2012;23:1533-9. [Crossref] [PubMed]
- Guo C, Yang B, Gu W, et al. Intravesical resiniferatoxin for the treatment of storage lower urinary tract symptoms in patients with either interstitial cystitis or detrusor overactivity: a meta-analysis. PLoS One 2013;8:e82591. [Crossref] [PubMed]
- Payne CK, Mosbaugh PG, Forrest JB, et al. Intravesical resiniferatoxin for the treatment of interstitial cystitis: a randomized, double-blind, placebo controlled trial. J Urol 2005;173:1590-4. [Crossref] [PubMed]
- Gao Y, Liao L. Intravesical injection of botulinum toxin A for treatment of interstitial cystitis/bladder pain syndrome: 10 years of experience at a single center in China. Int Urogynecol J 2015;26:1021-6. [Crossref] [PubMed]
- Manning J, Dwyer P, Rosamilia A, et al. A multicentre, prospective, randomised, double-blind study to measure the treatment effectiveness of abobotulinum A (AboBTXA) among women with refractory interstitial cystitis/bladder pain syndrome. Int Urogynecol J 2014;25:593-9. [Crossref] [PubMed]
- Nickel JC, Moldwin R, Lee S, et al. Intravesical alkalinized lidocaine (PSD597) offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU Int 2009;103:910-8. [Crossref] [PubMed]
- Madersbacher H, van Ophoven A, van Kerrebroeck PE. GAG layer replenishment therapy for chronic forms of cystitis with intravesical glycosaminoglycans--a review. Neurourol Urodyn 2013;32:9-18. [Crossref] [PubMed]
- Parsons CL, Greenspan C, Mulholland SG. The primary antibacterial defense mechanism of the bladder. Invest Urol 1975;13:72-8. [PubMed]
- Cervigni M, Natale F, Nasta L, et al. A combined intravesical therapy with hyaluronic acid and chondroitin for refractory painful bladder syndrome/interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:943-7. [Crossref] [PubMed]
- Porru D, Leva F, Parmigiani A, et al. Impact of intravesical hyaluronic acid and chondroitin sulfate on bladder pain syndrome/interstitial cystitis. Int Urogynecol J 2012;23:1193-9. [Crossref] [PubMed]
- Lai MC, Kuo YC, Kuo HC. Intravesical hyaluronic acid for interstitial cystitis/painful bladder syndrome: a comparative randomized assessment of different regimens. Int J Urol 2013;20:203-7. [Crossref] [PubMed]
- Lv YS, Zhou HL, Mao HP, et al. Intravesical hyaluronic acid and alkalinized lidocaine for the treatment of severe painful bladder syndrome/interstitial cystitis. Int Urogynecol J 2012;23:1715-20. [Crossref] [PubMed]
- Nickel JC, Egerdie B, Downey J, et al. A real-life multicentre clinical practice study to evaluate the efficacy and safety of intravesical chondroitin sulphate for the treatment of interstitial cystitis. BJU Int 2009;103:56-60. [Crossref] [PubMed]
- Nickel JC, Egerdie RB, Steinhoff G, et al. A multicenter, randomized, double-blind, parallel group pilot evaluation of the efficacy and safety of intravesical sodium chondroitin sulfate versus vehicle control in patients with interstitial cystitis/painful bladder syndrome. Urology 2010;76:804-9. [Crossref] [PubMed]
- Nickel JC, Hanno P, Kumar K, et al. Second multicenter, randomized, double-blind, parallel-group evaluation of effectiveness and safety of intravesical sodium chondroitin sulfate compared with inactive vehicle control in subjects with interstitial cystitis/bladder pain syndrome. Urology 2012;79:1220-4. [Crossref] [PubMed]
- Cervigni M, Natale F, Nasta L, et al. Intravesical hyaluronic acid and chondroitin sulphate for bladder pain syndrome/interstitial cystitis: long-term treatment results. Int Urogynecol J 2012;23:1187-92. [Crossref] [PubMed]
- Parsons CL, Housley T, Schmidt JD, et al. Treatment of interstitial cystitis with intravesical heparin. Br J Urol 1994;73:504-7. [Crossref] [PubMed]
- Davis EL, El Khoudary SR, Talbott EO, et al. Safety and efficacy of the use of intravesical and oral pentosan polysulfate sodium for interstitial cystitis: a randomized double-blind clinical trial. J Urol 2008;179:177-85. [Crossref] [PubMed]
- Daha LK, Lazar D, Simak R, et al. The effects of intravesical pentosanpolysulfate treatment on the symptoms of patients with bladder pain syndrome/interstitial cystitis: preliminary results. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:987-90. [Crossref] [PubMed]
- Tyagi P, Kashyap M, Hensley H, et al. Advances in intravesical therapy for urinary tract disorders. Expert Opin Drug Deliv 2016;13:71-84. [Crossref] [PubMed]
- Tyagi P, Chancellor M, Yoshimura N, et al. Activity of different phospholipids in attenuating hyperactivity in bladder irritation. BJU Int 2008;101:627-32. [Crossref] [PubMed]
- Peters KM, Hasenau D, Killinger KA, et al. Liposomal bladder instillations for IC/BPS: an open-label clinical evaluation. Int Urol Nephrol 2014;46:2291-5. [Crossref] [PubMed]
- Chuang YC, Lee WC, Lee WC, et al. Intravesical liposome versus oral pentosan polysulfate for interstitial cystitis/painful bladder syndrome. J Urol 2009;182:1393-400. [Crossref] [PubMed]
- Zhang QH, Shen XC, Zhou ZS, et al. Decreased nanobacteria levels and symptoms of nanobacteria-associated interstitial cystitis/painful bladder syndrome after tetracycline treatment. Int Urogynecol J 2010;21:103-9. [Crossref] [PubMed]
- Toft BR, Nordling J. Recent developments of intravesical therapy of painful bladder syndrome/interstitial cystitis: a review. Curr Opin Urol 2006;16:268-72. [Crossref] [PubMed]


