
The role of adipose stroma in prostate cancer aggressiveness
Resistance of prostate cancer to treatment remains a challenge. Surgery, chemotherapy, and androgen deprivation therapy cure most patients with early-stage disease. However, metabolic reprogramming, androgen receptor bypass, and sustained immunotolerance results in treatment-refractory prostate cancer that progresses to metastatic and lethal stages. Adverse outcome of prostate cancer, as well as of other cancers, is associated with obesity, a medical condition resulting from expansion of white adipose tissue (WAT). The normal function of WAT is to store or release lipids in response to physiological cues. Studies in mouse models have shown that WAT, which becomes inflamed, fibrotic and dysfunctional in obesity, is sufficient to enhance cancer progression irrespective of diet. Periprostatic WAT, more abundant in obese patients, plays a particularly important role in prostate cancer. Invasion of cancer cells into periprostatic WAT is a hallmark of prostate tumor aggressiveness (1).
Emerging evidence suggests that WAT-derived cells contribute to the population of cancer-associated fibroblasts (CAFs) that stimulate cancer cells. Adipocytes, the lipid-storing cells of WAT, differentiate from mesenchymal stromal cells (MSC) termed adipose stromal cells (ASCs). Mesenchymal stroma is the key constituent of a tumor microenvironment that promotes cancer progression in animal models. We have demonstrated that ASCs, abundant in WAT, are further expanded in obesity, become mobilized, and migrate to tumors, most likely from adjacent WAT depots (2). Adipocytes at the invasive tumor front undergo lipolysis and also appear to play an important role in cancer progression (1), in part, as a source of fatty acids utilized by cancer cells (3). In unpublished studies, we have shown that adipocytes can completely lose lipid droplets and de-differentiate back into stromal cells that are also recruited by tumors (Figure 1). Cell trafficking from WAT to tumors in obese prostate cancer patients is linked with poor survival (2).
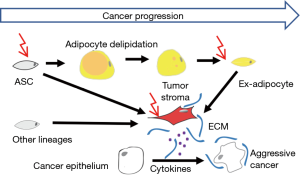
The molecular mechanisms through which ASCs promote cancer progression are multifaceted (Figure 1). Accumulating evidence indicates that ASCs operate through paracrine activation of angiogenic, immunosuppressive, anti-apoptotic, and mitogenic signaling. Adipokines secreted by adipocytes and ASC, recruit macrophages that also contribute to carcinoma progression, and mute T-cell anti-tumor immune response in part by sustaining the immune checkpoint (3). Chemokines secreted by ASCs also directly signal to carcinoma cells and promote their aggressiveness. Specifically, CXCL12 appears to play an important role in prostate tumor growth and invasiveness (4). Some of the cancer-promoting effects of ASCs may be contact-dependent. ASCs remodel extracellular matrix (ECM) enabling tumor desmoplasia and secrete tumor-trophic factors. The ability of adipose cells to promote metastatic dissemination of cancer has been reported (3). There is also emerging evidence that WAT-derived cells contribute to therapy resistance of various cancers.
A key function of CAFs appears to be their ability to induce epithelial-mesenchymal transition (EMT) of carcinoma cells (3). While the role of EMT in metastatic dissemination is debated, acquisition of the ‘cancer stem cell’ properties and resistance to therapy is undoubtedly a hallmark of EMT. Confirming that ASCs acquire the role of CAFs in tumors, our recent publication demonstrated the role of ASCs in inducing EMT in prostate cancer (5). We showed that obesity promotes EMT in cancer cells and tumor invasion into the periprostatic WAT. Co-culture of human prostate cancer cells with periprostatic ASCs resulted in EMT marker upregulation and increased invasiveness of cancer cells. Importantly, upon ASC exposure, prostate cancer cells were found to become more resistant to docetaxel, cabazitaxel, and cisplatin. We confirmed these findings in mice grafted with prostate tumors. ASCs protect cancer cells from chemotherapy at least in part through controlling oxidative damage: while an increase in reactive oxygen species (ROS) was observed in prostate cancer cells treated with cisplatin, it was blunted in ASC co-culture (5). In other studies, WAT-derived cells were found to decrease mitochondrial respiration while increasing glycolysis. A similar metabolic symbiosis may take place in prostate cancer although further work is needed to confirm this possibility.
The challenge of cancer medicine is to develop approaches that lead to inactivation of CAFs, including those derived from adipose cells. Through screens of combinatorial libraries, we have identified a marker of ASCs and a probe that recognizes them in both WAT and tumors (6). Cytotoxic hunter-killer peptides, D-WAT and D-CAN, have been developed for targeted ablation of mouse and human ASCs using this marker. Importantly, as our study demonstrated, D-CAN suppressed the ASC-dependent EMT and cancer progression and potentiated cisplatin efficacy in human cell culture and in animal models (5). This publication has drawn considerable interest reflected in subsequent editorials (7-10). As commented by Wade and Kyprianou, if EMT induction is the primary role of ASCs then EMT-suppressing agents should have comparable efficacy. Indeed, they have found that blockade of TGF-β signaling synergizes with the antiandrogen enzalutamide (7). Paternoster and Falasca point out that it will be important to reconcile how individual cellular components of WAT, including adipocytes, endothelial cells, and infiltrating immune cells, contribute to prostate cancer (8). Bhagirath and Saini reiterate that it remains to be determined if ASCs and adipocytes converge on similar pathways in impacting EMT or if these cell types act via distinct mechanisms (9). It is not currently known whether ASC-depleting agents target other stromal cell populations previously reported to be important in the tumor microenvironment (10). Another question raised is whether ASC depletion, preventing the EMT, can be effective against cancers that are already aggressive (10). This issue is important because disseminated cancer cells may lose their dependency on ASC. In addition to these nuances on the role of ASC, there are many remaining unanswered questions regarding the obesity-cancer link in general. The complexity of WAT engagement in cancer progression needs to be better understood as we advance towards personalized treatment strategies. While many questions remain, the current take-home message is that dosing of chemotherapy and immunotherapy needs to be tailored for patients depending on their visceral adiposity.
Acknowledgments
Funding: The authors would like to acknowledge NIH grant R01 CA196259 (to J DiGiovanni and MG Kolonin), R21 CA216745 (to MG Kolonin) and the Harry E. Bovay, Jr. Foundation gift (to MG Kolonin).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Laurent V, Guerard A, Mazerolles C, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun 2016;7:10230. [Crossref] [PubMed]
- Zhang T, Tseng C, Daquinag AC, et al. CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumor microenvironment. Nat Commun 2016;7:11674-90. [Crossref] [PubMed]
- Lengyel E, Makowski L, DiGiovanni J, et al. Cancer as a Matter of Fat: The Crosstalk between Adipose Tissue and Tumors. Trends Cancer 2018;4:374-84. [Crossref] [PubMed]
- Saha A, Ahn S, Blando J, et al. Proinflammatory CXCL12-CXCR4/CXCR7 signaling axis drives Myc-induced prostate cancer in obese mice. Cancer Res 2017;77:5158-68. [Crossref] [PubMed]
- Su F, Ahn S, Saha A, et al. Adipose stromal cell targeting suppresses prostate cancer epithelial-mesenchymal transition and chemoresistance. Oncogene 2019;38:1979-88. [Crossref] [PubMed]
- Daquinag AC, Tseng C, Zhang Y, et al. Targeted Pro-apoptotic Peptides Depleting Adipose Stromal Cells Inhibit Tumor Growth. Mol Ther 2016;24:34-40. [Crossref] [PubMed]
- Wade CA, Kyprianou N. Adipose tissue: enabler of prostate cancer aggressive behavior. Transl Androl Urol 2019;8:S242-5.
- Paternoster S, Falasca M. Targeting the adipose tissue to ght prostate cancer. Transl Androl Urol 2019;8:S229-31.
- Bhagirath D, Saini S. Coping with chemoresistance in prostate cancer—co-targeting of adipose stromal cells? Transl Androl Urol 2019;8:S250-3.
- Arendt LM. Taking aim at a challenging target in pre-clinical models of prostate cancer. Transl Androl Urol 2019;8:S88-90. [Crossref] [PubMed]


