
Ureteroscopy in patients with spinal cord injury: outcomes from a spinal injury unit and a review of literature
Introduction
Spinal cord injury (SCI) patients represent a small but significant population in the management of urolithiasis, with the risk of stone development being between 7–20% over an 8- to 10-year period (1,2), and the peak onset occurring within the first 3–6 months following injury (1,3). This phase of stone formation is likely due to ‘immobilisation hypercalciuria’ secondary to lower limb bone demineralisation (4). Further phases of stone formation are typically due to chronic infection from urease-producing bacteria (2).
The sequela of SCI can also result in detrimental changes to the bladder that culminate in an increased risk of urolithiasis. Detrusor-sphincter dyssynergia, a common feature, can result in increased detrusor pressure which over time can lead to fibrosis, causing decreased bladder compliance and vesico-ureteric reflux (5,6). These combined factors, along with stasis of urine, recurrent urinary tract infection and hypercalciuria, can all lead to an increase in the development of urolithiasis (2).
In the general population stone free rates (SFR) following ureteroscopy have been reported between 77–93% after a single procedure, with stone size, number and location impacting on outcomes (7-9). However, to date, there are limited reports of the outcomes of ureteroscopy in the SCI population (10-13). We therefore report our experience at a regional SCI unit for ureteroscopy in upper tract stone disease in patients with SCI.
Methods
This audit was approved by Salisbury District Hospital. We retrospectively collected data at a single regional SCI facility from 2005–2017 for SCI patients who underwent ureteroscopy for stone disease. Patients with other causes of neurogenic bladder or with an age under 16 years were excluded. Demographic information, operative techniques, stone analysis, complications and follow up were reviewed. SFR and recurrence rate (RR) were determined by post-operative imaging which included X-ray (XRKUB), ultrasound scan (USS) and CT of the Kidneys, Ureter and Bladder (CTKUB). Preoperative assessment of stone burden was completed with CTKUB. Stone size was calculated through the sum of the longest linear measurement of all stones. Complications were reported using the Clavien Dindo classification (14).
Operative technique
All patients had pre-operative urine cultures sent. Where culture positive results were present pre-operative antibiotic courses were completed. All patients were given a single dose gentamicin (3 mg/kg) at induction unless pre-operative urine cultures dictated otherwise.
All procedures were completed under general anaesthesia with the patient either in the supine or lithotomy position dependent on lower limb contracture. Ureteral access and guidewire insertion were achieved urethrally in most patients; access via mitrofanoff or suprapubic tracts was required in some patients and performed using a flexible cystoscopy. Patients with ileal conduits required conduitoscopy using a flexible cystoscope. Access sheaths were used depending on operator preference. A flexible ureteroscope was then introduced over the guidewire using fluoroscopic guidance. Stone fragmentation was completed using a 200 or 270 U Ho:YAG laser fiber at 0.8 Hz/8 J. Where possible the fragments were retrieved with stone basket and sent for analysis. Double J stent placement at the end of the operation was based on surgeon preference.
The majority of SCI patients were followed up in the spinal injury clinic every 12–18 months with clinical review and imaging (USS and XRKUB). More frequent follow-up was at the discretion of the operating surgeon. Patients were classified as stone free provided residual fragments were less than 2 mm.
Results
Eight urological surgeons experienced with stone disease in SCI patients performed 41 procedures on 21 patients throughout this time, with one surgeon completing a quarter of all procedures. Mean age of patients was 49 years (range, 17–69 years) with a male to female ratio of 6:1. Level of spinal injury was cervical (71%) and thoracic (29%) with American Spinal Injury Association (ASIA) classification A (86%), C (9%) and D (5%) (15). Bladder management varied throughout the patient cohort. The majority of patients had a suprapubic catheter (SPC) in situ (34%). Other management included sheath catheter (SC) (24%), mitrofanoff ± augmented bladder (22%) intermittent catheterisation (IC) (10%), ileal conduit (7%), and indwelling urethral catheter (IUC) (2%) (Table 1).
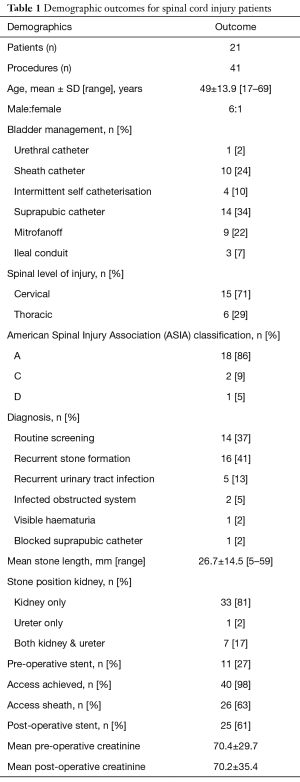
Full table
Mean stone length was 27 mm (range, 5–59 mm) demonstrating the generalised increased stone burden of SCI patients, particularly with its increased propensity within the kidney (98%). Stones were present in the kidney alone in 81%, in both the ureter and kidney in 17% of cases, and one patient had a stone solely in the ureter. Pre-operative stents were present in 27%, reflecting the nature of diagnosis as stone burden is typically identified through surveillance rather than acute presentation. Access was achieved in 98% of cases, with an access sheath being used in 63% (Table 1).
The overall SFR was 47%, defined as <2 mm fragments determined by follow-up XRKUB, US or CTKUB. RR was high at 42%, with a median follow-up period of 46 months. Seven repeat ureteroscopy cases were planned as a multistage approach due to the level of stone burden. The overall complication rate was 24%, all being Clavien Dindo grade 2 including urinary tract infection (n=2), sepsis/infection (n=3), lower respiratory tract infection (n=2), autonomic dysreflexia (n=1), pelvic collection (n=1), and laser failure (n=1). No patient required admission to the intensive care unit for post-operative complications. Median length of stay was 1 day (range, 0–9 days). A degree observation following intervention is essential in the majority cases to ensure that patients do not develop sepsis or autonomic dysreflexia, and to allow for early stent removal (Table 2).
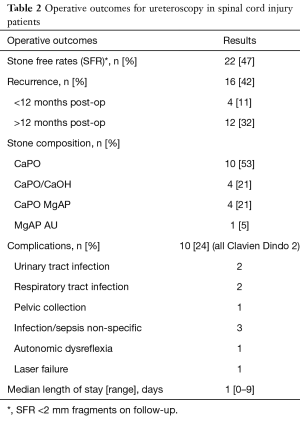
Full table
Stone analysis was completed in 19 cases with the majority of stones composed of purely calcium phosphate (53%). Others stones were composed of calcium phosphate/calcium oxalate mix (21%), calcium phosphate/magnesium ammonium phosphate mix (21%), and magnesium ammonium phosphate/ammonium urate mix (5%) (Table 2).
Comparison to studies in current literature
Four studies have reported outcomes for ureteroscopy in SCI patients, all being retrospective, completed over several years and containing small numbers of patients (Table 3) (10-13). While two studies limited their sample to SCI patients alone, Tepeler and Christman also included patients with other causes of neurogenic bladder such as cerebral disorders including stroke and multiple sclerosis, however, they did not differentiate between groups in their results (10,13).
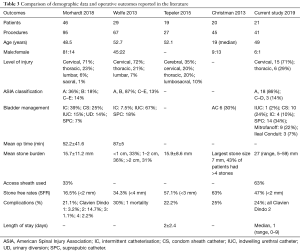
Full table
In total 114 patients underwent 234 procedures, with an average of 2 procedures per patient, which echoes our findings. Overall there were varying degrees of bladder management with most patients using either intermittent catheterisation, having an IUC, or a SPC. A small number of patients had undergone urinary diversion. Stone burden on average was between 15.7–15.9 mm which is significantly less than in our presented cohort of SCI patients being 27 mm. Our level of SFR (47%) appears to be in keeping with that currently reported of 16.5–63% despite having a greater mean stone burden than other published series (10-13).
The definition of SFR varied significantly between studies and ranged from 2–4 mm. It has been proposed that ‘zero fragment’ SFR is a more appropriate end point in patients with SCI, due to the inability to clear remaining stone fragments due immobility in many patients (16). In our practice we aim to remove as many fragments as possible during ureteroscopy, potentially reflected by our higher degree of access sheath use compared to other published series.
Published complication rates for SCI patients ranges from 21–30% (10-13). Whilst our review only found Clavien Dindo grade 2 complications, more significant complications have been seen in the literature including admission to the intensive care unit for urosepsis and respiratory failure. Wolfe et al. also noted one death following ureteroscopy due to a combination of these factors (12).
Discussion
Aggressive management for kidney stone disease in SCI is recommended due to potential loss of renal function (1), increased risk of infection, potential rapid progression of stone disease, and prevention of acute and sometimes insidious presentation of an infected obstructed system (2). It is therefore essential individuals with SCI undergo management of their stone burden in an appropriate and timely manner. Pre-operative optimisation and post-operative care should be planned, and risk factors negated where possible (17).
Presentation of SCI patients with urolithiasis can be challenging, particularly those with high SCI above T6. While recurrent urinary tract infection is the most typical presentation, one fifth of patients present acutely with urosepsis and hydronephrosis, which can lead to autonomic dysreflexia (2). Currently the UK recommends that the upper tracts for SCI patients should be monitored on a yearly basis, however there is no reported role for CT surveillance (18). Our series has demonstrated the value of screening as 36.8% of patients were diagnosed with urolithiasis through surveillance imaging, and a further 42.1% through monitoring following intervention and recurrence. Only two patients presented with an infected obstructed system that required urgent intervention. Surveillance is key to early detection and monitoring allowing for a planned multidisciplinary approach to any endourological intervention. This, alongside early optimisation of bladder management aids in the reduction and identification of stone disease (2,18).
Percutaneous nephrolithotomy (PCNL) remains the gold standard of treatment for stones >2 cm in diameter, with reported SFR in SCI patients of 62–96% (19,20). However, many patients require re-look procedures in order to achieve SFR of over 90% (2). While PCNL may be recommended for larger stones due to its superior SFR, it is not always technically possible, particularly in patients with SCI where anatomy can be distorted, with low muscle mass due to contractures.
Ureteroscopy offers a less invasive alternative to PCNL that may be able to achieve acceptable outcomes although at the cost of lower SFRs. The mean cumulative stone size in our patient group was 27 mm, with an SFR of 47%, demonstrating a large stone burden can be managed through ureteroscopy alone. Eight patients from our series had planned multi-stage procedures reflecting the need for careful consideration of the most suitable intervention.
SCI has been identified as one of the leading risk factors for morbidity in the surgical management of stone disease (17). Baldea et al. demonstrated through multivariate analysis that SCI is an independent predictor for increased rate of complication and mortality for PCNL with a mortality rate of 4.8% (21). They found the risk of post-operative sepsis and pneumonia was two-fold higher than that of a matched population (21). Overall major complication rates for PCNL range from 7–20% in SCI patients, with the most frequently reported complications being urosepsis, pneumothorax, perirenal abscess, and respiratory arrest (22). Ureteroscopy offers a less invasive modality than PCNL for the management of stone disease, however, it is not without risk. Consistent with our series complication rates in the literature vary from 21–30% and one death has been reported. Complications include urinary tract infection, respiratory tract infection, urosepsis, hypotension, and respiratory failure (10-13).
Special considerations for SCI patients
Upper tract access can be technically difficult, particularly in patients with lower limb contractures and reconstructed urinary tracts. It is essential that every effort is made to ensure the patient is stone free aiming for a “zero fragment” end point. This is particularly important in patients with a high SCI level as immobility makes it less likely that residual fragments will pass. Access sheath catheters can facilitate the removal of residual fragments and we are increasingly using these in SCI patients to try and achieve a zero-fragment end point. Our centre typically attempts to avoid leaving a urethral stent for prolonged periods as they may cause dysreflexia. If a stent is required, it is typically left on a tether and provided the patient is well, with no signs of sepsis, it is removed 24–72 hours post operatively.
Pre-operative cultures are essential in SCI patients given the high rates of post-operative sepsis. In our series pre-operative urine cultures were positive in 16 cases, with three having multi-resistant bacteria. This is typically due to bacterial colonisation of indwelling catheters and reconstructed urinary tracts (2).
Treatment at a specialist SCI centre can facilitate good post-operative care as medical and nursing staff are experienced in treating SCI patients. It is important to promptly identify and manage dysreflexia, sepsis and any other complication in a timely manner. Meticulous nursing care is needed to prevent pressure sores and support other care needs such as bowel management.
Limitations
There are several limitations to this study. It is retrospective and therefore subject to observational and selection bias. As a tertiary referral centre for SCI patients it is possible that some patients attended local urology units with post-operative complications and thus late complications may therefore be under-reported. Our sample size is small and all cases carried out at a single unit. Larger prospective multi-centre observational studies may help to identify factors to predict complications and outcomes so that we can improve patient counselling and guide better practice.
Conclusions
The management of urolithiasis in SCI patients is challenging and requires a multidisciplinary approach by teams experienced in treating SCI patients. Ureteroscopy provides a useful treatment option for patients with SCI, but SFR are inferior to those in the general population. Meticulous pre- and post-operative care is essential to prevent and manage the increased complication rate with this patient group.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This was an audit that was completed at Salisbury Hospital with the approval and registration of the audit. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Chen Y, DeVivo MJ, Roseman JM. Current trend and risk factors for kidney stones in persons with spinal cord injury: a longitudinal study. Spinal Cord 2000;38:346-53. [Crossref] [PubMed]
- Ramsey S, McIlhenny C. Evidence-based management of upper tract urolithiasis in the spinal cord-injured patient. Spinal Cord 2011;49:948-54. [Crossref] [PubMed]
- Hansen RB, Biering-Sorensen F, Kristensen JK. Urinary calculi following traumatic spinal cord injury. Scand J Urol Nephrol 2007;41:115-9. [Crossref] [PubMed]
- Naftchi NE, Viau AT, Sell GH, et al. Mineral metabolism in spinal cord injury. Arch Phys Med Rehabil 1980;61:139-42. [PubMed]
- Klausner AP, Steers WD. The neurogenic bladder: an update with management strategies for primary care physicians. Med Clin North Am 2011;95:111-20. [Crossref] [PubMed]
- García-Nieto V, Siverio B, Monge M, et al. Urinary calcium excretion in children with vesicoureteral reflux. Nephrol Dial Transplant 2003;18:507-11. [Crossref] [PubMed]
- Fabrizio MD, Behari A, Bagley DH. Ureteroscopic management of intrarenal calculi. J Urol 1998;159:1139-43. [Crossref] [PubMed]
- Grasso M. Ureteropyeloscopic treatment of ureteral and intrarenal calculi. Urol Clin North Am 2000;27:623-31. [Crossref] [PubMed]
- Sofer M, Watterson JD, Wollin TA, et al. Holmium:YAG laser lithotripsy for upper urinary tract calculi in 598 patients. J Urol 2002;167:31-4. [Crossref] [PubMed]
- Tepeler A, Sninsky BC, Nakada SY. Flexible ureteroscopic laser lithotripsy for upper urinary tract stone disease in patients with spinal cord injury. Urolithiasis 2015;43:501-5. [Crossref] [PubMed]
- Morhardt DR, Hadj-Moussa M, Chang H, et al. Outcomes of Ureteroscopic Stone Treatment in Patients With Spinal Cord Injury. Urology 2018;116:41-6. [Crossref] [PubMed]
- Wolfe T, Klausner AP, Goetz LL, et al. Ureteroscopy with laser lithotripsy for urolithiasis in the spinal cord injury population. Spinal Cord 2013;51:156-60. [Crossref] [PubMed]
- Christman MS, Kalmus A, Casale P. Morbidity and efficacy of ureteroscopic stone treatment in patients with neurogenic bladder. J Urol 2013;190:1479-83. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34:535-46. [Crossref] [PubMed]
- Ghani KR, Wolf JSJ. What is the stone-free rate following flexible ureteroscopy for kidney stones? Nat Rev Urol 2015;12:281-8. [Crossref] [PubMed]
- Whitehurst L, Jones P, Somani BK. Mortality from kidney stone disease (KSD) as reported in the literature over the last two decades: a systematic review. World J Urol 2019;37:759-76. [Crossref] [PubMed]
- Abrams P, Agarwal M, Drake M, et al. A proposed guideline for the urological management of patients with spinal cord injury. BJU Int 2008;101:989-94. [Crossref] [PubMed]
- Symons S, Biyani CS, Bhargava S, et al. Challenge of percutaneous nephrolithotomy in patients with spinal neuropathy. Int J Urol 2006;13:874-9. [Crossref] [PubMed]
- Rubenstein JN, Gonzalez CM, Blunt LW, et al. Safety and efficacy of percutaneous nephrolithotomy in patients with neurogenic bladder dysfunction. Urology 2004;63:636-40. [Crossref] [PubMed]
- Baldea KG, Blackwell RH, Vedachalam S, et al. Outcomes of percutaneous nephrolithotomy in spinal cord injury patients as compared to a matched cohort. Urolithiasis 2017;45:501-6. [Crossref] [PubMed]
- Nabbout P, Slobodov G, Mellis AM, et al. Percutaneous Nephrolithotomy in Spinal Cord Neuropathy Patients: A Single Institution Experience. J Endourol 2012;26:1610-3. [Crossref] [PubMed]


