
Outpatient buccal mucosal graft urethroplasty outcomes are comparable to inpatient procedures
Introduction
Urethral stricture disease is a relatively uncommon condition affecting 0.6–1.4% of the male population in the United States, but its negative effects on quality of life are well-described (1,2). Most urethral strictures are treated via endoscopic dilation or incision, but this carries a high failure rate and need for repeat endoscopic intervention or formal urethroplasty. Recent trends suggest a shift toward earlier treatment with urethroplasty, which has a higher reported success rate (3).
With a higher volume of urethroplasty being performed nationwide, increased attention has focused on optimizing the perioperative course and minimizing costs. Urethroplasty has traditionally been performed on an inpatient basis, with an average length of stay of 2.5 days (4). Several recent series suggest that outpatient urethroplasty has comparable outcomes and complication rates (5-9), yielding obvious cost savings implications for the healthcare system. However, these studies are limited by small patient populations and heterogeneous surgical techniques (anastomotic, substitution, etc.).
Traditionally, buccal mucosal graft (BMG) substitution urethroplasty patients have been admitted—at least overnight—for pain control and theoretical concern about impaired graft imbibition and inosculation with early physical activity. Recent data supports safe, early discharge in this population (7,9). We sought to analyze outcomes in our BMG urethroplasty population, comparing those repairs performed on an inpatient with those done in an outpatient setting. We hypothesized that surgical success and complications would be similar between the two groups.
Methods
After institutional review board approval, we performed a retrospective review of 1,230 patients who underwent urethroplasty by a single reconstructive urologist from 2007–2017. Of those, 143 underwent single stage or first stage BMG urethroplasty and were included in the analysis. Patients under 18 years old, second stage procedures, anastomotic procedures, perineal urethrostomy, penile skin flap urethroplasty and patients with less than 12 months of follow-up were excluded. Demographic, clinical, operative, and outcomes data were analyzed, comparing those who underwent inpatient versus outpatient management. Outpatient was defined as discharge without an overnight stay, while inpatient was defined as patients who had at least an overnight stay, including 23-hour observation (“Short Stay”).
Urethroplasty success was defined as functional voiding without need for further endoscopic or open reoperative management. Follow-up was calculated from the date of surgery to the date of data collection (6/27/2018). Complications were captured by EMR review and classified according to the Clavien-Dindo system.
In addition to surgical outcomes, we also evaluated for any differences in post-operative clinical encounters after a transition to outpatient BMG urethroplasty. A retrospective review of the medical record identified clinical encounters including telephone call, electronic messages, emergency room visits and hospital re-admissions. Data regarding the frequency of these visits was collected at 3-month interval timepoints over the course of the first year following urethroplasty.
Surgical technique
BMG harvest was performed using a standardized technique in both cohorts (10). No lingual grafts or double buccal harvests were performed. The patient receives perioperative cefazolin and gentamicin to protect against oral and genitourinary flora. An oral self-retaining Denhart retractor is placed in the mouth, exposing and stretching the oral mucosa. Three silk stay sutures are placed just inside of the vermillion border of the lip to aid in retraction. Stenson’s duct is identified and marked to avoid injury. The desired graft size is marked and injected with lidocaine with epinephrine for preliminary hydrodissection and hemostasis. Stay sutures are placed into the corners as the graft is harvested, taking care to avoid Stenson’s duct. Hemostasis is obtained with an epinephrine-soaked gauze and electrocautery. Oral mucosa donor sites were closed with interrupted 3-0 Chromic sutures. The graft is then defatted using Metzenbaum scissors while draped over a gloved finger and kept in sterile saline until placement.
Graft placement technique is dependent on stricture characteristics such as length, caliber and location. We prefer a two-team approach to BMG urethroplasty, where the urethra team informs the buccal team of the exact BMG dimensions required, but the harvest is initiated based on preoperative imaging. For first stage BMGs, the patient is seen in clinic on POD#5, at which time his mineral oil-soaked bolster dressing is removed. Patients are discharged home with prophylactic antibiotics, bladder antispasmodics and oral mouthwash including viscous lidocaine. Depending upon the urethroplasty technique, the silicone catheter remains in place for 2–4 weeks, at which time voiding cystourethrogram is performed. Three months following catheter removal, patients are evaluated with urinary flow rate and symptom score. Patients are then seen on an annual basis or as needed.
Statistical analysis
The Wilcoxon rank sum tests was used to determine differences in success rates between the inpatient and outpatient groups. A multivariable logistic regression model was used to test for significant predictors of urethroplasty failure. All analyses were carried out using JMP™ 13.0 by SAS (Cary, NC).
Results
Demographics
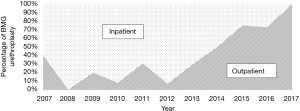
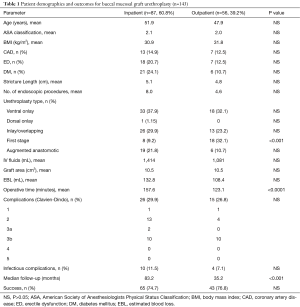
Over the course of the study period, 1,230 urethroplasties were performed at our institution by a single surgeon. Of these 143 patients (11.6%) who underwent BMG urethroplasty during the study period, 87 cases (60.8%) were performed on an inpatient/short stay basis and 56 (39.2%) on an outpatient basis. Over the course of the study period, our practice has shifted toward same-day discharge for BMG substitution urethroplasty (Figure 1). Patient characteristics were similar between inpatient and outpatient, including mean stricture length (5.1 vs. 4.8 cm), mean age (51.9 vs. 47.9 years), ASA classification (2.1 vs. 2.0), BMI (30.9 vs. 31.8 kg/m2), prevalence of coronary artery disease (14.9% vs. 12.5%), diabetes mellitus (24.1% vs. 10.7%), erectile dysfunction (20.7% vs. 12.5%) and mean number of prior endoscopic procedures (8.0 vs. 4.6) (Table 1).
Full table
Perioperative characteristics and outcomes
Mean operative time was slightly longer in the inpatient group (157.6 vs. 123.1 min, P<0.0001). Estimated blood loss was similar in the inpatient and outpatient groups (132.8 vs. 108.4 mL, P=0.06), as was mean intraoperative IV fluid administration (1,414 vs. 1,081 mL, P=0.9). Graft area was identical between the two groups, at 10.5 cm2 each (P=1.0). Surgical success was comparable in the two groups (74.7% inpatient vs. 76.8% outpatient, P=0.7), though median follow-up was longer in the inpatient group compared to the outpatient group (83.2 vs. 35.2 months, P<0.001).
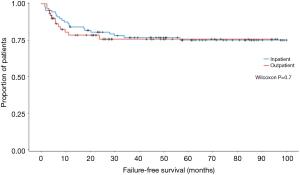
A greater proportion of first stage graft repairs were performed as an outpatient compared to inpatient (69% vs. 31%, P<0.001). Rates of major, minor, and overall complications were similar between groups (29.9% inpatient vs. 26.8% outpatient, P=0.7). Additionally, infectious complications (cellulitis, abscess, UTI, etc.) were similar between the inpatient and outpatient cohorts (11.5% vs. 7.1%, P=0.4). Kaplan-Meier analysis of failure-free survival demonstrates no significant difference between the two groups (Figure 2). Our analysis also found no significant difference between the two groups in frequency of unplanned post-operative clinical encounters consisting of telephone calls, electronic messages, emergency room visits, and hospital re-admissions at 3, 6, and 12 months.
On multivariate logistic regression analysis, multiple parameters analyzed as possible predictors of graft failure (age, CAD, DM, smoking, ED, stricture length, and number of prior endoscopic procedures) were not predictive of failure. After controlling for potential confounders, failure was not predicted by inpatient versus outpatient surgery.
Discussion
The present study describes our cumulative experience with outpatient BMG urethroplasty, providing further evidence supporting the safety of this practice. We found no difference in surgical success comparing inpatient and outpatient urethroplasty (74.7% vs. 76.8%, respectively) and no difference in complications. A 2017 series of 118 consecutive anterior urethroplasties, of which 78 (66%) were BMG urethroplasty, also found similar success between inpatient and outpatient settings (79% vs. 89%, respectively) (9). To our knowledge, the present study is the largest to exclusively compare outcomes of BMG urethroplasty patients in the two care settings.
Outpatient urethroplasty: surgical outcomes
A 2002 study on outpatient urethroplasty also found comparable surgical success between inpatient and outpatient cases, with success rates of 88% and 93%, respectively (5). Urethroplasty techniques in this 2002 study were varied, with 50% of patients undergoing excision and primary anastomosis (EPA), 30% had penile skin flap, and 17% underwent BMG urethroplasty. In 2005, McDonald et al. reported similar outcomes in their series of outpatient urethroplasty—38% of which were BMG urethroplasty—with success rates of 94% and 97%, respectively (6).
The mean stricture length in our series is nearly twice that reported in other studies on outpatient urethroplasty (6). In our practice, we tend to favor EPA urethroplasty when feasible, as success rates are high and the morbidity of BMG harvest is avoided. As such, we typically reserve BMG urethroplasty for long or recalcitrant strictures, which are inherently higher risk for recurrence. We believe that this variation in practice patterns in the utilization of substitution urethroplasty accounts for the lower surgical success rate demonstrated in our data. Though stricture characteristics and operative techniques vary, all studies confirm that urethroplasty—including BMG urethroplasty—can be performed safely and effectively in the outpatient setting.
Outpatient BMG urethroplasty: patient reported outcomes
While a mounting body of evidence supports the safety and efficacy of outpatient urethroplasty, there is a paucity of data regarding patient reported outcome measures (PROM). With reimbursement often now more closely tied to patient satisfaction (11), ensuring adequate patient teaching, understanding and comfort prior to expedited discharge is essential. A number of groups are developing urethroplasty-specific patient centered outcome measures, but these do not currently incorporate discharge timing (12).
In 2015, Okafor and Nikolavsky described PROM of 48 patients undergoing both inpatient and outpatient anterior urethroplasty (7). Within 24 hours of discharge, patients were administered a validated health-related quality of life (HRQoL) questionnaire, including an assessment of discharge timing. Patients in both groups reported similar HRQoL metrics. Rates of satisfaction with discharge timing in outpatient and short stay patients were 86% and 90%, respectively. As outpatient urethroplasty becomes more commonplace, patient reported outcomes remain a fertile area of investigation in order to better illuminate patient experience and satisfaction.
Possible barriers to outpatient BMG urethroplasty
A potential impediment to outpatient BMG urethroplasty is the concern for impaired graft take with early mobility, especially in patients undergoing first stage urethroplasty. In these cases, the graft may be subject to external shear forces and displacement. Randomized studies evaluating graft take in first stage urethroplasty between the two settings have not been performed. In the plastic surgery literature, a trend towards more outpatient skin grafting and early mobilization is noted, and evidence suggests that graft take is comparable to inpatient procedures and infection rates may be lower in the outpatient setting (13,14). Erpelding et al. reported success with outpatient skin grafting for repair of acquired buried penis further illustrating the safety and efficacy of ambulatory reconstructive procedures (15).
To minimize the risk of graft loss in first stage procedures, we aggressively quilt the graft, secure a mineral oil-soaked bolster with suture from the graft edges, and immobilize the penis against the patient’s abdomen with adhesive gauze wrap. The same bolster design is used in the inpatient setting as well. Patients are advised to minimize activity at home for 5 days, and then the dressing is taken down in clinic by our dedicated nurse. We have had excellent results with this practice, even on an outpatient basis. For one stage inlay and onlay grafts, the risk of graft mobility in the early healing process is likely low, as the graft is usually well fixed to the urethral edges, quilted to the underlying tissue, and further immobilized by pressure from the Foley catheter. Indeed, ample data in the early outpatient urethroplasty studies demonstrate good outcomes in the BMG substitution groups.
Another potential concern with BMG urethroplasty is bleeding from the graft harvest site and harvest site pain. All patients are discharged home with narcotics for pain management. However, we have found graft harvest site bleeding to be exceedingly rare, and usually when it occurs it is managed in the recovery room by applying pressure to the bleeding area. A review of the literature confirms the rarity of this complication at about 1% (16). In our series, only one patient experienced a delayed bleed on POD#3, requiring a clinic visit and buccal pressure with a gauze sponge with timely resolution. Randomized trials studying oral pain and morbidity associated with BMG site closure suggest varied recommendations for site management but universally report relatively low morbidity of BMG harvest overall (17-21). With judicious hemostasis, partial closure of the graft site with interrupted 3-0 chromic sutures, and inspection of the harvest site in the operating room, bleeding after BMG urethroplasty is rare and should not preclude performance in an outpatient setting.
Limitations
Our study has potential limitations inherent in the design of any retrospective study. Follow-up time was calculated from the date of the operation to the point of data collection. This was based on the assumption that the patient may not follow-up if doing well, but would return if symptoms recurred. Our follow-up assumption does not account for patients who possibly sought care elsewhere, but this potential bias should affect all patients equally and not substantively change conclusions of the analysis. This follow-up calculation is commonplace among tertiary centers where patients may travel a great distance for care (9).
Because inpatient and outpatient cohorts were not randomized, a selection bias for younger, healthier patients may occur for the outpatient procedure, thereby improving outcomes. However, no differences existed in any major comorbidities between the inpatient and outpatient groups. The BMG urethroplasty cohort in our study was heterogeneous, as there were some single stage and some first stage procedures performed. However, between the two cohorts, there were no intentional differences in surgical technique or perioperative care.
Our definition of surgical success—a lack of further intervention—was standard across both inpatient and outpatient cases, but differs from other studies, which utilize cystoscopy or imaging to determine success (22,23). However, there are compliance and logistic issues involved in repeated office cystoscopy in patients after urethroplasty, and we have found that patients with a urethra as small as 10F can often void and empty well. As such, we rely on patient symptoms rather than cystoscopy or imaging to dictate the need for further intervention.
Conclusions
Outpatient BMG urethroplasty can be performed safely without increased complications or compromised outcomes. With expedited discharge, thorough preoperative counseling and post-operative instruction is essential to maximize operative success and patient satisfaction. Based on our experience, we now reserve inpatient BMG urethroplasty for patients with significant medical comorbidity that warrant post-anesthesia observation.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. AF Morey receives honoraria for being a guest lecturer/meeting participant for Boston Scientific and Coloplast Corp. The other authors declare that they have no relevant financial interests.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of The University of Texas Southwestern Medical Center at Dallas (ID: 102012-021).
References
- Lubahn JD, Zhao LC, Scott JF, et al. Poor quality of life in patients with urethral stricture treated with intermittent self-dilation. J Urol 2014;191:143-7. [Crossref] [PubMed]
- Lucas ET, Koff WJ, Rosito TE, et al. Assessment of satisfaction and quality of life using self -reported questionnaires after urethroplasty: a prospective analysis. Int Braz J Urol 2017;43:304-10. [Crossref] [PubMed]
- Liu JS, Hofer MD, Oberlin DT, et al. Practice patterns in the treatment of urethral stricture among american urologists: a paradigm change? Urology 2015;86:830-4. [Crossref] [PubMed]
- Blaschko SD, Harris CR, Zaid UB, et al. Trends, utilization, and immediate perioperative complications of urethroplasty in the United States: data from the national inpatient sample 2000-2010. Urology 2015;85:1190-4. [Crossref] [PubMed]
- Lewis JB, Wolgast KA, Ward JA, et al. Outpatient anterior urethroplasty: outcome analysis and patient selection criteria. J Urol 2002;168:1024-6. [Crossref] [PubMed]
- MacDonald MF, Al-Qudah HS, Santucci RA. Minimal impact urethroplasty allows same-day surgery in most patients. Urology 2005;66:850-3. [Crossref] [PubMed]
- Okafor H, Nikolavsky D. Impact of short-stay urethroplasty on health-related quality of life and patient's perception of timing of discharge. Adv Urol 2015;2015:806357. [Crossref] [PubMed]
- MacDonald S, Haddad D, Choi A, et al. Anterior urethroplasty has transitioned to an outpatient procedure without serious rise in complications: data from the national surgical quality improvement program. Urology 2017;102:225-8. [Crossref] [PubMed]
- Theisen K, Fuller TW, Bansal U, et al. Safety and surgical outcomes of same-day anterior urethroplasty. Urology 2017;102:229-33. [Crossref] [PubMed]
- Morey AF, McAninch JW. Technique of harvesting buccal mucosa for urethral reconstruction. J Urol 1996;155:1696-7. [Crossref] [PubMed]
- Stanowski AC, Simpson K, White A. Pay for performance: are hospitals becoming more efficient in improving their patient experience? J Healthc Manag 2015;60:268-85. [Crossref] [PubMed]
- Breyer BN, Edwards TC, Patrick DL, et al. Comprehensive qualitative assessment of urethral stricture disease: toward the development of a patient centered outcome measure. J Urol 2017;198:1113-8. [Crossref] [PubMed]
- Southwell-Keely J, Vandervord J. Mobilisation versus bed rest after skin grafting pretibial lacerations: a meta-analysis. Plast Surg Int 2012;2012:207452. [Crossref] [PubMed]
- Tallon BG, Oliver GF. Comparison of inpatient bed rest and home convalescence following split thickness skin grafting to the lower leg. Australas J Dermatol 2007;48:11-3. [Crossref] [PubMed]
- Erpelding SG, Hopkinds M, Dugan A, et al. Outpatient surgical management for acquired buried penis. Urology 2019;123:247-51. [Crossref] [PubMed]
- Markiewicz MR, DeSantis JL, Margarone JE, et al. Morbidity associated with oral mucosa harvest for urological reconstruction: an overview. J Oral Maxillofac Surg 2008;66:739-44. [Crossref] [PubMed]
- Wood DN, Allen SE, Andrich DE, et al. The morbidity of buccal mucosal graft harvest for urethroplasty and the effect of nonclosure of the graft harvest site on postoperative pain. J Urol 2004;172:580-3. [Crossref] [PubMed]
- Muruganandam K, Dubey D, Gulia AK, et al. Closure versus nonclosure of buccal mucosal graft harvest site: A prospective randomized study on post operative morbidity. Indian J Urol 2009;25:72-5. [Crossref] [PubMed]
- Rourke K, McKinny S, St Martin B. Effect of wound closure on buccal mucosal graft harvest site morbidity: results of a randomized prospective trial. Urology 2012;79:443-7. [Crossref] [PubMed]
- Wong E, Fernando A, Alhasso A, et al. Does closure of the buccal mucosal graft bed matter? Results from a randomized controlled trial. Urology 2014;84:1223-7. [Crossref] [PubMed]
- Soave A, Dahlem R, Pinnschmidt HO, et al. Substitution urethroplasty with closure versus nonclosure of the buccal mucosa graft harvest site: A randomized controlled trial with a detailed analysis of oral pain and morbidity. Eur Urol 2018;73:910-22. [Crossref] [PubMed]
- Erickson BA, Elliott SP, Voelzke BB, et al. Multi-institutional 1-year bulbar urethroplasty outcomes using a standardized prospective cystoscopic follow-up protocol. Urology 2014;84:213-6. [Crossref] [PubMed]
- Erickson BA, Ghareeb GM. Definition of successful treatment and optimal follow-up after urethral reconstruction for urethral stricture disease. Urol Clin North Am 2017;44:1-9. [Crossref] [PubMed]


