Comparison of 3.5 cm and transcorporal cuffs in high-risk artificial urinary sphincter populations
Introduction
Over forty years after its introduction, the artificial urinary sphincter (AUS) remains the gold standard for treatment of stress incontinence in men with nearly 90% of patients demonstrating improvement in quality of life (1). However, almost a third of patients with an AUS may require revision surgery within 5 years for mechanical failure, poor functional outcomes, infection, or urethral cuff erosion (2-4). A number of factors have been identified which predispose to complication and need for revision surgery after AUS implantation including radiation, prior urethral surgery, hypogonadism, and urethral atrophy.
Numerous surgical techniques have been utilized to address these issues including cuff downsizing, cuff relocation, tandem cuff placement, urethral buttressing, and transcorporal (TC) cuff placement. TC cuff placement was first described in 2002 and has been used in the revision setting for patients with urethral atrophy and high-risk patients with history of prior erosion or urethroplasty (5,6). In 2010, the 3.5 cm cuff was introduced to enable downsizing and address the issue of incomplete coaptation in patients with urethral atrophy. While there were early concerns about an increased risk of erosion, the rates of erosion observed thus far with the 3.5 cm cuff appear to be similar to larger cuff sizes (6).
Despite the promise of these techniques, there have been few large studies investigating outcomes of AUS in high-risk patients. We sought to evaluate outcomes from over ten years in managing incontinent men with comprised urethras. We compared operative success and erosion events between men undergoing TC and 3.5 cm cuff cases and examined potential factors associated with adverse outcomes in high-risk AUS patients.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the medical records of all patients who underwent AUS placement at our tertiary referral center by a single surgeon between 2007 and 2018 with a minimum of 6 months of follow-up. We identified patients with a potential for urethral compromise as defined by the use of a 3.5 cm cuff or TC cuff placement. In our practice, men with urethral atrophy were treated with a 3.5 cm cuff and those with prior urethroplasty or erosion were managed with placement of a TC cuff. Patient demographics and clinical factors such as history of coronary artery disease (CAD), prior urethral erosion or urethroplasty, hypertension, radiation, erectile dysfunction (ED), prior incontinence or ED surgery were tabulated, as were surgical details such as cuff size and surgical technique (i.e., standard or TC cuff placement).
AUS candidates were assessed preoperatively by history (including average pad count) and physical exam. Cystoscopy was performed and non-invasive urodynamics including post-void residual was obtained in all men with history of obstructive voiding symptoms, bladder neck contracture, urethral stricture, or prior urethral surgery (e.g., prior sling, AUS, urethroplasty). Urinary continence was determined by history at follow-up visits, and patients were deemed socially continent if they reported using ≤1 pad/day.
All 3.5 cm cuffs were implanted using a uniform perineal surgical approach for cuff placement (7). TC cuff placement was performed as previously described and was performed in cases where previous erosion or urethral surgery prevented safe dissection between the corpus spongiosum and the corpora cavernosa (5). All TC patients were impotent, nearly impotent, or were not concerned about impact on erectile function. Cuff erosion was confirmed using cystoscopy before and/or during the time of surgical explant.
Demographic and erosion outcome data were compared based on cuff technique. Statistical significance was considered at P≤0.05, and reported P values are 2 sided. All analysis was done with SPSS® version 17.0. This study was approved by the University of Texas Southwestern Medical Center institutional review board.
Results
Demographics
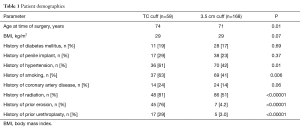
During the study period from 2007 to 2018, a total of 625 AUS cases were performed, of which 59 (9%) used a TC technique and 168 (27%) used a 3.5 cm cuff (Table 1). Median age amongst men undergoing TC cuff placement was 74 years and 71 in men undergoing 3.5 cm cuff placement. Patients in both groups presented with a similar rate of comorbid factors that predispose to vascular compromise and known risk factors of urethral erosion including diabetes and prior penile implant placement.
Full table
History of radiation (81% vs. 51%, P<0.00001) tended to be higher among men who received TC cuff compared to those who received the 3.5 cm cuff. A larger number of patients in the TC group had had prior urethral cuff erosion than the 3.5 cm group (76% vs. 4.2%, P<0.00001). Similarly, men in the TC cohort had a greater number of prior urethroplasties in comparison with the 3.5 cm cuff population (29% vs. 3.0%, P<0.00001). Demographic characteristics that are associated with increased vascular disease such as CAD, smoking, and hypertension were all significantly higher in the TC patient cohort compared with the 3.5 cm cuff group (CAD 24% vs. 14%, P=0.06; smoking 63% vs. 41%, P=0.006; hypertension 61% vs. 42%, P=0.01).
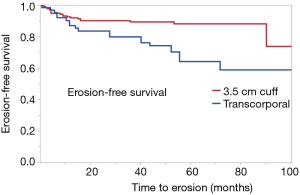
Cuff erosion
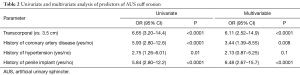
A total of 53 cuff erosions occurred over a median follow-up time of 49 months. Of these, 28 (52.8%) occurred in men with TC cuff placement and 25 (47.2%) in men with a 3.5 cm cuff (Figure 1). We found that 47% of patients with TC cuff placement had an erosion event compared with 15% of men with 3.5 cm cuff placement. On Kaplan-Meier analysis, patients with TC cuff placement had poorer erosion-free survival compared to men those with 3.5 cm cuff (P<0.001). The TC population had a shorter median time to erosion, compared with men in the 3.5 cm cuff cohort [4.4 vs. 9.5 months, respectively (P=0.02)]. On univariate analysis, a TC cuff was associated with increased odds of erosion (OR 6.65, 95% CI: 3.20–14.4, P<0.0001) when compared with a 3.5 cm cuff (Table 2). On multivariate analysis, TC cuffs remained associated with significantly increased odds of cuff erosion (Table 2). Similarly, known risk factors for cuff erosion such as CAD (a marker for vascular compromise) and prior penile implantation also increased odds of urethral cuff erosion.
Full table
Discussion
In this study, we present over a decade of experience in managing incontinence in men with atrophied or compromised urethra. This study represents one of the largest contemporary cohorts of high-risk men undergoing AUS placement (5,7-12). Over long-term follow-up, we found that the TC cuff has a significantly higher rate of urethral erosion when used in salvage procedures, such as men with prior urethral surgery and erosion. Our findings demonstrate that the 3.5 cm cuff continues to provide excellent outcomes in terms of continence and patient safety.
In addition to concern for poor tissue integrity in an atrophic urethra, replacement of AUS in high-risk patients is problematic due to the need to elevate the urethra off of the corpora cavernosa. In men with prior erosion or urethroplasty, this surgical plane is potentially obliterated or fibrotic, raising the risk of urethral injury and further vascular compromise of the urethra. In order to address this concern, alternate methods to circumferentially compress the urethra without posterior dissection of the urethra were developed.
TC cuff placement was originally described by Guralnick and colleagues as a salvage technique to improve outcomes in the revision setting for patients with urethral atrophy or those high-risk patients with prior erosion (5). In their original series, 31 men (5 with prior urethroplasty or erosion) had placement of a TC cuff, and all reported excellent continence with no erosion events over a median follow-up of 17 months. Other groups have subsequently reported outcomes from TC cuff placement in high-risk AUS patients. For instance, the Trauma and Urologic Reconstructive Network of Surgeons (TURNS) recently published data from their series of 18 men who had a TC cuff placed following urethral erosion and concurrent urethral repair. These men were followed for a median period of 22 months, and the authors observed an erosion rate of 24% (12).
Compared with prior studies, the rate of erosion in our TC population is significantly higher, at 45%. This difference is likely attributed to our exclusive use of the TC cuff for patients with prior erosion rather than for those with urethral atrophy. Furthermore, our median follow-up of 49 months is significantly longer than other series in the literature. The Kaplan-Meier analysis in the present study demonstrates that many erosion events happen later in the life, suggesting that with longer follow-up more adverse events can be appropriately captured.
Traditionally, atrophy has been managed with cuff relocation or urethral bulking using local tissue or xenograft or TC cuff placement. However, the introduction of the 3.5 cm cuff by American Medical Systems allowed for more appropriate urethral sizing for men with atrophy. Some in the urological community have raised concern about increased risk of erosion from a smaller cuff, particularly in men with urethral atrophy (13-15). However, we have previously demonstrated that the 3.5 cm cuff has no significant difference in erosion events compared to larger cuff sizes (6,16). Rather, the majority of erosion events in men with 3.5 cm cuff occurred in those with a history of pelvic radiation, suggesting the erosion is attributed to poor underlying tissue quality and not the cuff. In this challenging population, thorough pre-operative counseling is crucial, taking care to weigh the potential risk of increased erosion with the desire for continence. McGeady et al. observed similar findings in their smaller cohort of men with 3.5 cm cuff implantation, where those with failure or erosion were considered high-risk having had prior erosion, prior urethroplasty, or AUS explantation (13). This present study reaffirms that the 3.5 cm cuff is safe and effective in men whose spongiosal measurements indicate a small urethra, and we continue to use the device as our primary management of urethral atrophy.
The increased risk of erosion in our high-risk TC cuff population is likely attributable to inherent changes in urethral vascularity after prior erosion or transection during urethroplasty. The underlying scar tissue impedes longitudinal blood flow along the course of the urethra, and prior urethral dissection reduces the availability of collateral perfusion (4,13). Additionally, the TC cuff is placed more distally along the urethra than the standard cuff. Here, the corpus spongiosum is less robust than at the proximal bulbar urethra and provides less support to protect the urethra from erosion (17).
Despite the dorsal support of the corporal tissue, the chronic ventral compression of the spongiosum by a TC cuff—in the setting of poor urethral perfusion—leads to a significantly increased risk of erosion in these patients. As such, we reserve the TC cuff for select, high-risk patients and prefer the 3.5 cm for those patients with pure atrophy of the spongiosum. While the TC cuff can restore continence in high-risk patients, it should not be viewed as panacea. Prior to TC cuff implantation, patients should be counseled regarding the risk of AUS replacement in the context of their surgical history and prior complications.
Conclusions
TC cuff placement in patients with a compromised urethra—such as those with prior urethral erosion or urethroplasty—is associated with an elevated risk for subsequent erosion events. The 3.5 cm cuff continues to demonstrate safety and efficacy in men with demonstrated urethral atrophy. Prior to AUS placement in these high-risk patients, appropriate counseling should be provided to patients regarding the risk of removal or revision surgery.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. Allen F. Morey receives honoraria for being a guest lecturer/meeting participant for Boston Scientific and Coloplast Corp. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This was a study in which we solely retrospectively reviewed the patients’ medical records, so informed consent was not needed. This study (102012-019) was approved by the Institutional Review Board at UT Southwestern.
References
- Viers BR, Linder BJ, Rivera ME, et al. Long-Term Quality of Life and Functional Outcomes among Primary and Secondary Artificial Urinary Sphincter Implantations in Men with Stress Urinary Incontinence. J Urol 2016;196:838-43. [Crossref] [PubMed]
- Dalkin BL, Wessells H, Cui H. A national survey of urinary and health related quality of life outcomes in men with an artificial urinary sphincter for post-radical prostatectomy incontinence. J Urol 2003;169:237-9. [Crossref] [PubMed]
- Linder BJ, Rivera ME, Ziegelmann MJ, et al. Long-term Outcomes Following Artificial Urinary Sphincter Placement: An Analysis of 1082 Cases at Mayo Clinic. Urology 2015;86:602-7. [Crossref] [PubMed]
- Raj GV, Peterson AC, Toh KL, et al. Outcomes following revisions and secondary implantation of the artificial urinary sphincter. J Urol 2005;173:1242-5. [Crossref] [PubMed]
- Guralnick ML, Miller E, Toh KL, et al. Transcorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophy. J Urol 2002;167:2075-8; discussion 2079. [Crossref] [PubMed]
- McKibben MJ, Shakir N, Fuchs JS, et al. Erosion rates of 3.5-cm artificial urinary sphincter cuffs are similar to larger cuffs. BJU Int 2019;123:335-41. [Crossref] [PubMed]
- Hudak SJ, Morey AF. Impact of 3.5 cm artificial urinary sphincter cuff on primary and revision surgery for male stress urinary incontinence. J Urol 2011;186:1962-6. [Crossref] [PubMed]
- Lee D, Zafirakis H, Shapiro A, et al. Intermediate outcomes after transcorporal placement of an artificial urinary sphincter. Int J Urol 2012;19:861-6. [Crossref] [PubMed]
- Blah M, Caremel R, Sibert L, et al. Treatment of male urinary incontinence by artificial urinary sphincter with intracavernous cuff. Prog Urol 2008;18:114-9. [Crossref] [PubMed]
- Aaronson DS, Elliott SP, McAninch JW. Transcorporal artificial urinary sphincter placement for incontinence in high-risk patients after treatment of prostate cancer. Urology 2008;72:825-7. [Crossref] [PubMed]
- Wiedemann L, Cornu JN, Haab E, et al. Transcorporal artificial urinary sphincter implantation as a salvage surgical procedure for challenging cases of male stress urinary incontinence: surgical technique and functional outcomes in a contemporary series. BJU Int 2013;112:1163-8. [Crossref] [PubMed]
- Keihani S, Chandrapal JC, Peterson AC, et al. Outcomes of Urethroplasty to Treat Urethral Strictures Arising From Artificial Urinary Sphincter Erosions and Rates of Subsequent Device Replacement. Urology 2017;107:239-45. [Crossref] [PubMed]
- McGeady JB, McAninch JW, Truesdale MD, et al. Artificial urinary sphincter placement in compromised urethras and survival: a comparison of virgin, radiated and reoperative cases. J Urol 2014;192:1756-61. [Crossref] [PubMed]
- Brant WO, Erickson BA, Elliott SP, et al. Risk factors for erosion of artificial urinary sphincters: a multicenter prospective study. Urology 2014;84:934-38. [Crossref] [PubMed]
- Linder BJ, de Cogain M, Elliott DS. Long-term device outcomes of artificial urinary sphincter reimplantation following prior explantation for erosion or infection. J Urol 2014;191:734-8. [Crossref] [PubMed]
- Simhan J, Morey AF, Singla N, et al. 3.5 cm artificial urinary sphincter cuff erosion occurs predominantly in irradiated patients. J Urol 2015;193:593-7. [Crossref] [PubMed]
- Schlomer BJ, Dugi DD 3rd, Valadez C, et al. Correlation of penile and bulbospongiosus measurements: implications for artificial urinary sphincter cuff placement. J Urol 2010;183:1474-8. [Crossref] [PubMed]