
Reinforcing vasal suture technique improves sperm concentration and pregnancy rates in men undergoing vasovasostomy for vasectomy reversal
Introduction
Vasectomy reversals (VRs) have been performed for over 100 years, with the first known epididymovasostomy (EV) reported in 1902 (1). Early outcomes and patency rates were modest, in the 40% range, and remained stable until the introduction of microsurgical techniques in the 1970s (2). Although initial results with the new microsurgical approach were 71% with vasovasostomies (VV—already a landmark achievement), further refinements yielded patency rates as high as 74–98% (3,4).
Following the use of the operating microscope in the 1970s, several additional concepts have been introduced, including modified single-layered closures, vasal stents, and robotic-assisted repairs. Unfortunately, none of these new techniques have been able to further improve outcomes over the traditional, refined microsurgical approaches (5-7). Indeed, to our knowledge, other than minor variations in technique (e.g., intussuscepted EV) that have had a debatable impact on outcomes, there have not been any notable technical or device improvements that have been able to further optimize VR results for at least 30 years.
One of the challenges with improving VR outcomes is that on the surface, they appear to already be nearly perfect. As an example, some series report success rates of up to 98%, while online advertisements are up to 99.5% (3,8,9). However, nearly all VR publications use patency (>0 sperm) to define success, which likely results in a significant overestimation of true success rates. In an earlier analysis of our series of VR outcomes, we identified a 30% difference in success rates when using >0 sperm compared to >39 million (M) total sperm (10). These rates would likely be further impacted by inclusion of men undergoing VV only, those <12 years since vasectomy, and other similar factors. Given these limitations, the true rate of success with contemporary VR techniques is unknown.
In our tertiary referral practice, we had commonly performed revision VRs for men who had unsuccessful prior attempts. Over time, we noticed a clear pattern, wherein the far majority of cases appeared to have failed secondary to a separation of the vas deferens. Given this observation, we began performing a modified technique to provide additional anastomotic support. We hypothesized that the additional reinforcing vasal sutures (ReVas) would lead to reduced failure rates, higher sperm concentrations, and ultimately improved pregnancy rates. The objective of this manuscript was, therefore, to analyze outcomes of our cohort prior to and following implementation of the new technique.
Methods
Study cohort and surgeon
Following Institutional Review Board approval, a prospective, consecutive database has been maintained of all men undergoing VV or EV at our institution. From January 2014 through June 2019, a total of 200 men were treated with a VV or EV, of whom 169 sought a first-time VR and comprise the current cohort. Among these men, 69 had semen analysis data available to allow for comparative analyses. Men who underwent VV or EV for a secondary reversal, primary epididymal obstruction, or to bypass other causes of obstruction other than prior vasectomy were excluded from the current analysis.
All surgeries were performed at a single, high-volume VR center by the same surgical team. The primary surgeon was formally trained as a male fertility microsurgeon and had previously completed two microsurgery fellowships as well as a dedicated microsurgery training course.
Clinical evaluation and database
All men considering a reversal undergo a thorough history and physical examination, with demographics and clinical data obtained, including duration since vasectomy, prior paternity, prior inguinal hernia repairs or scrotal surgery, and partner age and fertility status. Additionally, examination is performed to assess for the presence of granulomas, testicular size, hydroceles, epididymal cysts, or other scrotal pathology. No laboratory or imaging tests are routinely obtained. All data are entered into a prospective registry, along with operative variables and follow-up data, including semen analysis and pregnancy outcomes.
Follow-up periods were defined both as the time since VR as well as the time between the reversal and most recent known outcome (either semen analysis or pregnancy status). It was recognized that neither measure represented an optimal definition for follow-up, as the former overestimates the true follow-up, while the latter underestimates it (i.e., successful pregnancy leads to no further queries).
Surgical technique and background
Beginning in January of 2018, our team introduced a modification to the traditionally-described VR technique (11). The surgical procedure is started in a similar manner to previously published methods, with the vasa brought through the skin incisions and dissected. Care is taken during dissection to avoid “stripping” the peri-vasal tissue away from the vas in order to provide adequate support tissue later in the procedure.
The decision for VV vs. EV is made at the time of vasal transection and is based fully on the presence or absence of sperm or sperm parts from the testicular vas. If no sperm are seen, additional cuts are made proximal to the testicle. If no sperm are seen despite 3–4 additional cuts and following additional waiting time, the decision is made to proceed with an EV. The vasal anastomosis is otherwise performed in a similar manner as has been previously described.
The VV is the performed in two layers, with 5–6, 10-0 nylon sutures placed in the lumen, and an additional 6–8, 8-0 nylon sutures placed in the adventitia. Once the VV has been completed, the vasa are brought closer together to approximate a region approximately 3 cm away from the anastomosis in each direction. At this point, the vasa are sutured together to achieve a shotgun-type, parallel configuration. A total of 5–10 sutures (or one running) are placed on each side of the vasa (if possible) using permanent suture (5-0 Prolene, C-1 taper). Although it is recognized that the peri-vasal tissue includes the deferential blood supply, no attempts are made to avoid capturing the vessels during suturing. Sutures are also placed as close to the vas as possible without penetrating the wall of the vas to provide additional support. See Figure 1 for a graphical depiction of the traditional and ReVas techniques.
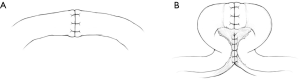
In the case of an EV procedure, the testicle is fully delivered, and an appropriate position for the anastomosis is identified on the epididymis. The vas is then freed up over a length of 5–6 cm, passed through the tunica vaginalis, and secured with 5-0 Prolene sutures. The vas is then passed parallel to the epididymis, with the cut end of the vas passing beyond the area of anticipated anastomosis. In another configuration (particularly for shorter vasa), the vas is passed perpendicular and beyond to the epididymis by approximately 2 cm. The peri-vasal tissue is then sutured to either the epididymis (in the first configuration) or the testicle (second configuration). Similar to the VV, 5–10 passes are made to adequately secure the vas with permanent suture (5-0 Prolene), which is then separated slightly from the peri-vasal tissue to circle back to the site of anastomosis.
Once the reinforcing sutures have been placed, the EV is performed in the traditional intussuscepted manner. Specifically, the epididymal sheath is incised, and an appropriate epididymal tubule is identified. The vas is then brought to the 12 o’clock position and secured to the epididymal sheath with an 8-0 suture. Two, double-armed 10-0 sutures are then placed in a parallel fashion in the epididymal tubule and the tubule incised. If sperm are present, the needles are pulled through and placed in 4 quadrants in the vasal lumen. The vas is then secured to the epididymal sheath at the 6 o’clock position with another 8-0 suture, and the 10-0 sutures are tightened and tied. Additional 8-0 sutures are then placed, and the testicle is returned to the normal anatomic position.
Outcomes and statistics
To assess outcomes, periodic attempts are made to contact all patients to query most recent semen analysis results and pregnancy status. Men who report achieving a successful pregnancy are no longer contacted.
The primary outcome for the study was sperm concentration using various definitions for success: >0/mL, >100,000/mL, >1 M/mL, >5 M/mL, and >15 M/mL. Five M was specifically selected based off of data by Majzoub and colleagues who demonstrated similar pregnancy rates at the >5 M/mL threshold (12). Only sperm concentration was used in the current study given the tertiary nature of our practice and difficulty in obtaining complete and reliable semen analysis data on all patients. Additionally, given the differing methods, techniques and overall reliability used by various labs to assess motility, morphology, and volume, concentration was felt to represent the most consistent measure. It was also selected based off the findings in the previously cited Majzoub study, where concentration demonstrated similar predictability when compared to other semen analysis measures (including total motile per ejaculate) (12).
Natural pregnancy rates between cohorts were more challenging to compare in a completely unbiased manner given the longer follow-up period in the ReVas (−) group. To mitigate these differences, pregnancy outcomes were queried in men who were >1 year but <2 years out since their reversal. These criteria were selected a priori to allow for an equal comparison between groups, as they provided a sufficient time period following surgery while controlling the period of follow-up. Despite these controls, results would slightly bias in favor of ReVas (−) given that a larger percentage of men would be closer to the 2-year mark, while the ReVas (+) men were closer to the 1-year time point (since the technique was introduced in Jan 2018).
To address potential bias of inflated pregnancy rates (due to self-reporting pregnancy rates in addition to phone queried outcomes), all men who were in the unknown status (unable to obtain follow-up information) were marked as pregnancy (−). Although this likely underestimates the true pregnancy rates, it was felt to provide a more accurate representation for comparison purposes and to allow for greater statistical power. This was also felt to be appropriate given that all men are encouraged at the time of surgery to contact our office if they were able to achieve a successful pregnancy.
Statistical comparisons were performed between men who underwent the ReVas technique [ReVas (+)] versus those in the traditional technique cohort [ReVas (−)]. Data were reported as medians/ranges in cases of non-normal distributions and mean ± standard deviations in normal parametric distributions. Statistical analyses included Pearson’s coefficient, likelihood ratios, Student’s t-tests, and Wilcoxon rank sum tests, where appropriate. For multivariate analyses, a generalized linear model was used with a binomial distribution. All tests were performed using JMP (SAS, Cary, NC, USA), with two-sided P values of <0.05 considered significant.
Results
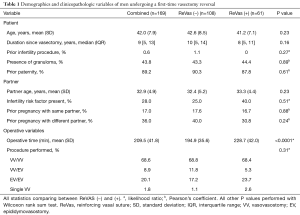
A total of 169 patients underwent a first-time VR at our institution from January 2014 through February 2019. Of these men, 61 received the new ReVas technique, while 108 underwent the traditional reversal. In reviewing baseline demographic and clinical information, there were no significant differences between cohorts regarding age, time since vasectomy, prior infertility procedures or paternity, presence of granulomas, partner age, partner infertility risk factors, or partner prior pregnancies. Operative time was significantly higher for the ReVas (+) group by an average of 34 minutes (P<0.0001), and there were no differences in the rate of VV vs. EV overall (P=0.31). See Table 1 for full clinical and demographic information between cohorts.
Full table
The median time of follow-up was 5.9 months, with ReVas (−) men having significantly longer follow-up due to the ReVas procedure being a newly introduced technique. The longer follow-up duration with the ReVas (−) men would be expected to bias nearly all results in favor of the ReVas (−) group. One potential exception would be with the variable, “most recent concentration” as the extended duration may hypothetically predispose towards a longer potential period to develop delayed stenosis, as highlighted in a recent meta-analysis (13). Pregnancy rates would also likely not be affected since attempts were made to compare outcomes within the first 2 years of the reversal as previously described.
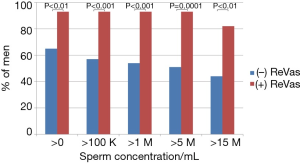
Regarding primary and secondary outcomes, men who underwent the ReVas technique demonstrated significant improvements in every primary and secondary outcome variable. Specifically, the percentage of men achieving >0/mL, >100,000/mL, >1 M/mL, >5 M/mL, or >15 M/mL were all notably higher in the ReVas (+) cohort. Similarly, the highest and most recent sperm concentrations and pregnancy rates were also significantly higher for the ReVas (+) group. See Table 2 for key outcomes between cohorts. The odds ratios for >0, >100,000/mL, >1 M/mL, >5 M/mL, >15 M/mL, and pregnancy with ReVas (+) were 7.0, 9.9, 11.1, 12.3, 5.8, and 8.1, respectively. See Table 2 and Figure 2 for a summary of key outcome measures between cohorts.

Full table
A subset analysis of men who underwent VV bilaterally with the ReVas technique demonstrated a 95% success rate when using a definition of >0, >100,000, >1 M, >5 M, and >15 M. In contrast, in ReVas (−) men, the success rate was 79%, 68%, 64%, 61%, and 54%, respectively. When at least one side was able to undergo a VV, results in the ReVas (+) group continued to be higher than ReVas (−) (>0 =93% vs. 69%; >100,000 =93% vs. 60%; >1 M =93% vs. 57%; >5 M =93% vs. 54%; >15 M =82% vs. 47%; >39 M =73% vs. 46%).
In reviewing ReVas (+) men who had a VV/EV compared to VV/VV, similar success rates were noted using definitions of >0/mL, >100,000/mL, >1 M/mL, and >5 M/mL, however, when using the definition of >15 M, statistically significant differences were noted. Specifically, 95% of VV/VV men achieved >15 M/mL compared to only 50% with VV/EV (P=0.01). The number of pregnancies were inadequate to perform subset analyses.
Among men who only underwent one VV (other side with EV or no repair), and despite small numbers of men with data available (n=14), the ReVas technique demonstrated significantly greater success rates with most definitions (>0/mL 86% vs. 29%, P=0.03; >100,000/mL 86% vs. 29%, P=0.03; >1 M/mL 86% vs. 29%, P=0.03; >5 M/mL 86% vs. 29%, P=0.03; >15 M/mL 43% vs. 17%, P=0.30). There were insufficient data to compare outcomes among men who had bilateral EVs or a single EV (n=3 between both cohorts).
Interestingly, in evaluating the impact of duration since vasectomy, in the ReVas (+) cohort, the time since vasectomy was not correlated with diminished outcomes (P=0.32 to 0.61). In contrast, in the ReVas (−) group, the time since reversal was significantly associated with the >0, >100,000, and >1 M groupings. The time since vasectomy was also significantly correlated with a need for EV on one or both sides (P<0.0001).
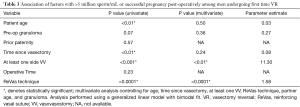
In evaluating the likelihood for either >5 M/mL or a pregnancy (success), univariate analysis demonstrated that patient age, time since vasectomy, at least one side VV, and ReVas technique were all significantly associated, with pre-op granuloma nearly reaching significance (P=0.07). In contrast, prior paternity and operative time were not correlated. On multivariate analysis controlling for age, time since vasectomy, at least one VV, ReVas technique, partner age, and granuloma, only the ReVas technique and the ability to perform at least one VV were associated with success. See Table 3 for univariate and multivariate outcomes.
Full table
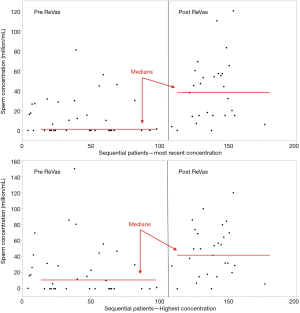
One concern with any sequential series is that learning curve effects may contribute to the outcomes. Figure 3 demonstrates both highest and most recent sperm concentration pre and post ReVas introduction. Results demonstrate an abrupt change in outcomes immediately after introduction of ReVas for both the highest (would favor cases with longer follow-up) and most recent (includes men with subsequent stenosis) sperm concentration, suggesting that findings are likely secondary to the ReVas technique rather than learning curve factors.
Discussion
The ReVas technique represents a significant advancement in VR surgery. Compared to men undergoing a traditional reversal, those who had a repair with the ReVas technique experienced significantly greater outcomes regardless of definition utilized: >0/mL, >100,000/mL, >1 M/mL, >5 M/mL, >15 M/mL, and highest and most recent concentrations. Additionally, pregnancy rates were approximately 8× higher in the ReVas (+) group. ReVas also represented a significant predictor of success, even after controlling for other pre-operative and intra-operative variables.
The underlying mechanism for the improved outcomes likely relates to the increase in tissue support to reduce partial or complete VV dehiscence. Although published data are limited on the topic, vasal separation represents a relatively common finding at the time of secondary reversal attempts. As there are no strong tissues nearby to which the vas can be sewn, and given the limited strength of serosal vasal stitches, the ReVas technique provides a greater surface area of tissue, thereby eliminating tension on the anastomosis. The additional tissue apposition may also provide improved neovascularity, which may also partly account for the higher sperm concentrations achieved over time.
The current study also highlights several other interesting findings that provide indirect evidence of improved outcomes with ReVas. The first is that the technique appears to negate the impacts of duration since vasectomy on overall outcomes. Among men who were ReVas (−), an increasing duration since reversal resulted in poorer outcomes. This finding may relate to softening of scar tissue over time that may lead to a robust repair among men who are further out from their vasectomy. The addition of the ReVas technique, therefore, may provide additional support and reduce the likelihood for failure in men who are further out from vasectomy. A second interesting finding is that men who had only one VV and were ReVas (−) had much worse outcomes compared to the single VV ReVas (+) group. And third, more stringent definitions utilized for success led to greater disparities between ReVas (+) and (−) men. Each of these findings, along with the notably different pregnancy rates, would suggest that the ReVas technique achieved a more robust repair compared to the traditional approach.
Findings also highlight how a “successful outcome” can vary dramatically based on the definition utilized. The most common definition used for success in the literature is “patency” among men undergoing VV, with most series including only first-time reversals and some also including time since vasectomy criteria. Importantly, using this very lenient definition, the ReVas (−) cohort would exhibit a success rate of 90%, which is consistent with other high-volume surgical series (3). However, if the criteria were broadened to an all-comer, first time reversal population that included VV and EV procedures and a definition of >15 M/mL on most recent semen analysis, the success rate would drop dramatically to 35% in the ReVas (−) cohort compared to 73% in the ReVas (+) group.
Defining success is also further complicated by the fact that true success (pregnancy and live birth) is highly dependent upon partner factors. To help clarify this issue, Majzoub and colleagues recently demonstrated that 5 M sperm/mL post reversal represented a key threshold, above which there were no notable differences in pregnancy outcomes (12). These data are consistent with our current series, which demonstrated that while there were no statistically significant differences between ReVas (+) and (−) using the most lenient of definitions, there were dramatic differences using all others. Additionally, pregnancy rates were 8× higher in the first 2 years among ReVas (+) men, which confirms that higher sperm concentrations are more likely to yield improved pregnancy outcomes. All of these data would suggest that the common definition of “patency” should no longer be considered an acceptable measure of success, but rather, a more stringent definition should be used, such as 5 M/mL (which correlates with pregnancy outcomes).
The current study has several notable limitations including its non-randomized nature, limited pregnancy data, short-term follow-up, and lack of complete semen analyses on all patients. Some of these limitations are mitigated by the sequential, prospective nature of the data and similar baseline clinical and demographic factors. Additionally, the current series represents a relatively large dataset, with key outcomes available on 69 men. As the data also represent a single-center, single-surgeon cohort, external validation is required to determine if these changes achieve similar results in other settings.
Implementation of the ReVas technique resulted in significant improvements in sperm concentration and an 8.1-fold increase in pregnancy rates within the first 2 years after reversal. Results were particularly pronounced when more strict definitions for a successful outcome were used and in cases of suboptimal conditions (single VV and longer duration since vasectomy). Although the technique added to the overall operative time, it represents an easily teachable procedure that could be implemented without advanced training. Given the single-surgeon nature of the current series, external validation is warranted.
Acknowledgments
Special thanks to Holli Burgon for her assistance with formatting and submission.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional review board of the Mayo Clinic (No. 14-009415). All patients had previously provided consent to release their records for medical research.
References
- Jequier AM. Edward Martin (1859-1938). The founding father of modern clinical andrology. Int J Androl 1991;14:1-10. [Crossref] [PubMed]
- Derrick FC Jr, Yarbrough W, D'Agostino J. Vasovasostomy: results of questionnaire of members of the American Urological Association. J Urol 1973;110:556-7. [Crossref] [PubMed]
- Namekawa T, Imamoto T, Kato M, et al. Vasovasostomy and vasoepididymostomy: Review of the procedures, outcomes, and predictors of patency and pregnancy over the last decade. Reprod Med Biol 2018;17:343-55. [Crossref] [PubMed]
- Silber SJ. Microscopic vasectomy reversal. Fertil Steril 1977;28:1191-202. [Crossref] [PubMed]
- Herrel LA, Goodman M, Goldstein M, et al. Outcomes of microsurgical vasovasostomy for vasectomy reversal: a meta-analysis and systematic review. Urology 2015;85:819-25. [Crossref] [PubMed]
- Jeon JC, Kwon T, Park S, et al. Loupe-Assisted Vasovasostomy Using a Prolene Stent: A Simpler Vasectomy Reversal Technique. World J Mens Health 2017;35:115-9. [Crossref] [PubMed]
- Kavoussi PK, Harlan C, Kavoussi KM, et al. Robot-assisted microsurgical vasovasostomy: the learning curve for a pure microsurgeon. J Robot Surg 2019;13:501-4. [Crossref] [PubMed]
- Patel SR, Sigman M. Comparison of outcomes of vasovasostomy performed in the convoluted and straight vas deferens. J Urol 2008;179:256-9. [Crossref] [PubMed]
- Belker AM, Thomas AJ Jr, Fuchs EF, et al. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol 1991;145:505-11. [Crossref] [PubMed]
- Alom M, Ziegelmann M, Savage J, et al. Office-based andrology and male infertility procedures-a cost-effective alternative. Transl Androl Urol 2017;6:761-72. [Crossref] [PubMed]
- Lipshultz LI, Rumohr JA, Bennett RC. Techniques for vasectomy reversal. Urol Clin North Am 2009;36:375-82. [Crossref] [PubMed]
- Majzoub A, Tadros NN, Polackwich AS, et al. Vasectomy reversal semen analysis: new reference ranges predict pregnancy. Fertil Steril 2017;107:911-5. [Crossref] [PubMed]
- Farber NJ, Flannigan R, Li P, et al. The Kinetics of Sperm Return and Late Failure Following Vasovasostomy or Vasoepididymostomy: A Systematic Review. J Urol 2019;201:241-50. [Crossref] [PubMed]


